
Behavioral Changes of Spilled Oil in the Marine Ecosystem
1 Clean
Nigeria Associates Limited/Gte 18 Ken Sarowiwa Road Port Harcourt, Rivers
State, Nigeria
|
|
ABSTRACT |
||
|
When oil is
spilled at sea it normally spreads out and moves on the sea surface under the
influence of the wind and current while undergoing a number of chemical and
physical changes. These processes are collectively termed weathering and
determine the behavior of the oil. An oil spill is the release of the liquid
petroleum hydrocarbon into the environment, especially marine areas, as a
result of human activity and other factors as equipment failures, human
errors, willful damage to equipment and oil installations etc. Oil is the
most common pollutant of the marine environment. The behavior of spilled oil
depends on the oil properties and the environmental conditions. It is very
important to recognize the dynamic nature of spilled oil because the
properties of spilled oil can change over time. It is important to monitor
the continuous changes in the properties of the spilled oil, as response
strategies may have to be modified to suit the current changes in oil
behavior. The properties of crude or refined oils vary in their physical and chemical
characteristics. These characteristics affect their volatility, toxicity,
weathering rate and persistency. Oil spills have a devastating and long term
impact on waterways and coastal areas around the world. Seabirds are
frequently affected by offshore oil spills. Spills can severely harm turtle
eggs and damage fish larvae, causing deformities. Shellfish and corals are
particularly at risk since they cannot escape the runaway slick. Oil spills
are also responsible for tainting algae, which perform a vital role in water
ecosystems. Oil spills can be partially controlled by chemical dispersion,
combustion, mechanical containment and adsorption. As the world advances
technologically, unfortunately accidents do happen and spills reoccur more
frequently than we would like. A good knowledge and understanding of the
types of oil, oil properties and the changes in the behavior of oil is very
critical in effective response planning, strategies choices, execution and
overall oil spill emergency response management in the marine ecosystems. |
|||
|
Received 08 May 2025 Accepted 10 June 2025 Published 09 July 2025 Corresponding Author Etuk
Etiese Akpan, etukcom@yahoo.com DOI 10.29121/IJOEST.v9.i4.2025.681 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Oil Spills, Marine,
Ecosystem, Environment, Behavior, Changes, Marine Oil Spills Oil Properties, Spilled
Oil Weathering, Ecological and Environmental Impacts |
|||
1. INTRODUCTION
An oil spill is the release of a liquid petroleum hydrocarbon into the environment, especially marine areas, due to human activity and is a form of pollution. Oil is the most common pollutant in the oceans. More than 3 million metric tons of oil contaminate the sea every year.
The majority of oil pollution in the oceans comes from land, runoff and waste from cities, industry and rivers carries oil into the ocean. Ships cause about a third of the oil pollution in the oceans when they wash out their tanks or dump their bilge water.
The kind of oil spill we usually think about is the accidental or intentional release of petroleum products into the environment as result of human activity (drilling, manufacturing, storing, transporting, waste management), that floats on the surface of water bodies as a discrete mass and is carried by the wind, currents and tides.
They have destructive effects on coastal ecosystems. Marine oil spill is a serious consequence of offshore oil drilling and its oceanic transportation.
Crude oil, and refined products, in the marine environment are subject to physical, chemical, and biological processes that change their composition and environmental impact.
The weathering of crude oil involves evaporation or volatilization, emulsification, dissolution, and oxidation (chemical, photo-, and microbial).
The horizontal transport or movement of crude oil is accomplished through spreading, advection, dispersion, and entrainment, whereas the vertical transport of oil involves dispersion, entrainment, Langmuir circulation, sinking, overwashing, partitioning, and sedimentation.
Crude oil from seeps and spills of persistent oils often form tar residues or tarballs that become stranded on the shoreline.
Conceptual and computer models aid in predicting the behavior and fate of oil and oil products in the marine environment.
The ultimate fate of oil and oil products in the environment depends on their composition, source, and persistence. Seeps, spills to surface water, deepwater subsea releases, and diffuse (non-point) sources behave in different ways.
2. Causes of Marine Oil Spills
A number of things cause oil spills in the marine environment, ranging from carelessness to deliberate dumping. Only a small percentage of global oil spills are related to tanker accidents such as explosions, hull failure,running aground, and collisions. One of the most common causes of oil spills is actually runoff from the land. Numerous land-based engines such as those used to run cars function on petroleum fuel and use petroleum based lubricants. All of these substances are slowly released, accumulating on roads and in the ground and ultimately ending up in the ocean. Oil spills can also be caused by natural seepage, especially in the ocean. As tectonic plates shift, they may release oil from reserves trapped Deep beneath the ocean floor. Natural seepage can also be accelerated through human activity such as drilling.
Figure 1
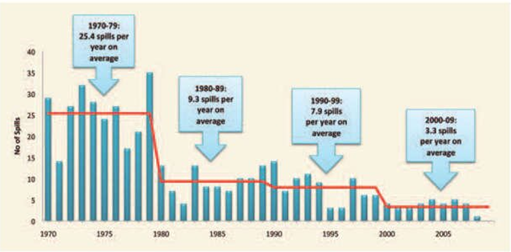
|
Figure 1 Number of Large Oil Spills Involving Releases of Over 700 Tonnes of Oil, Per Year Between 1970 and 2009 Worldwide (ITOPF 2010) |
Other causes include, deliberate acts by terrorists, acts of war, vandals or illegal dumping.
3. The Properties of Oil
Oil properties varies depending on the type of oil and their origins. The main physical properties that affect the behaviour and the persistence of an oil spilled at sea are specific gravity, distillation characteristics, vapour pressure, viscosity and pour point. All are dependent on chemical composition, such as the proportion of volatile components and the content of asphaltenes, resins and waxes.
4. Density (specific gravity)
Specific gravity is the measure of the density of the oil in relation to freshwater, whose density is 1 kg/m3, as a general rule, oils are low density. Density dictates the buoyancy of oil on water and it influences spreading and natural dispersion. The density of oil is expressed either in units of mass per unit volume (kg/m3).
5. Boiling point and boiling range
The rate at which oil evaporates is indicated by its initial boiling point and boiling range. The lower these are the faster evaporation will occur.
6. Viscosity
There is an inverse relationship between oil viscosity and oil movement on the water surface and there is direct relationship between oil viscosity and the surrounding temperature. When the oil is high in viscosity, it will move slowly, but when the oil viscosity is low, the oil movement is fast, depending on water temperature. Further, absorption of heat from the sun will affect the apparent viscosity of spilled oil.
Figure 2
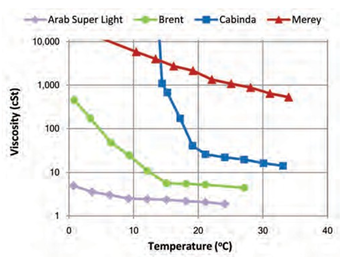
|
Figure 2 Viscosity/Temperature Relationship for the Four Crude Oil Types (ITOPF 2002) |
7. Pour point
The pour point is the formation of an internal micro crystalline structure of oil when the ambient temperature below the oil will not flow and it will behave as a solid.
8. Flashpoint (Volatility)
This is an important factor in relation to the safety of clean-up operations, because the flashpoint is the lowest temperature at which sufficient vapour exists above the spilled oil to yield a flammable mixture.
9. Solubility
Most oil components are soluble in water while others are not, but the more volatile components are the more soluble, which represents a significant toxicity to marine life when exposed.
10. Asphaltenes content
Asphaltenes is the main role in the formation and stability of water-in-oil emulsions; therefore, the low Asphaltenes oil is not stable emulsions.
11. Distillation Characteristics
In the distillation process, as the temperature of an oil is raised, different components reach their boiling point in succession, evaporate, and are then cooled and condense. The distillation characteristics are expressed as the proportions of the parent oil that distil within given temperature ranges.
12. Processes that Affect the Behavior of Spilled Oil
1)
Weathering
Following an oil spill or any other event that releases crude oil or crude oil products into the marine environment, weathering processes begin immediately to transform the materials into substances with physical and chemical characteristics that differ from the original source material (crude oil). The process of weathering involves individual processes such as spreading, evaporation, dispersion, dissolution, emulsification, biodegradation, photo-oxidation, sedimentation/sinking and stranding.
2)
Spreading
As soon as oil is spilled, it immediately starts to spread over the sea surface. The speed at which this takes place depends largely on the viscosity of the oil, temperature, waves, turbulence, tidal streams, currents and the volume spilled. And this is governed by the physiochemical parameters viscosity and density. Oil with low viscosity spread much faster than those with high viscosity. Liquid oils initially spread as a coherent slick but quickly begin to break up. As the oil spreads and the thickness reduces, its appearance changes from the black or dark brown coloration of thick oil patches to iridescent and silver sheen at the edges of the slick.
Figure
3
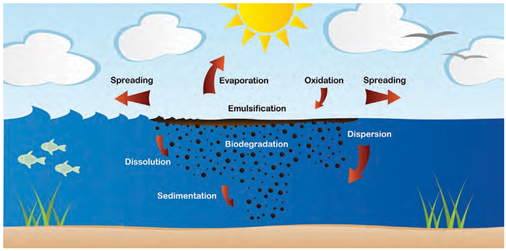
|
Figure 3 Weathering Processes Acting on Oil at Sea. (ITOPF 2002). |
3)
Evaporation
In many oil spills, evaporation is the most important process in terms of mass balance. Within a few days following a spill, light crude oils can lose up to 75 percent of their initial volume and medium crudes up to 40 percent. In contrast, heavy or residual oils will lose no more than 10 percent of their volume in the first few days following a spill. Stiver and Mackay (1984)
The more volatile components of an oil will evaporate to the atmosphere. The rate of evaporation is governed by the oil property volatility and depends on ambient temperatures and wind speed. In general, those oil components with a boiling point below 200°C will evaporate within a period of 24 hours in temperate conditions.
Figure
4

|
Figure 4 Evaporation of Lighter Oil (IOPF 2002). |
4)
Emulsification
Emulsification is the process of formation of various states of water in oil, often called “chocolate mousse” or “mousse” among oil spill workers. These emulsions significantly change the properties and characteristics of spilled oil. Stable emulsions contain between 60 and 85 percent water thus expanding the volume by three to five times the original volume of spilled material. The density of the resulting emulsion can be as great as 1.03 g/mL compared to a starting density ranging from about 0.95 g/mL to as low as 0.80 g/mL.
Emulsions form most readily for oils which, when spilled, have a combined Nickel/Vanadium concentration greater than 15ppm or an asphaltene content in excess of 0.5%. International Maritime Organization (2005). The rate of oil emulsification is greatly influenced by the percentage of asphaltene content in the oil.
Most significantly, the viscosity of the oil typically changes from a few hundred to a few hundred thousand milli Pascal-seconds, a typical increase of three orders of magnitude. This increase in viscosity can change a liquid petroleum product into a heavy, semi-solid material Fingas et al. (1996).
5)
Dissolution
Dissolution is the chemical stabilization of oil components in water. Dissolution accounts for only a small portion of oil loss, but it is still considered an important behavior parameter because the soluble components of oil, particularly the smaller aromatic compounds, are more toxic to aquatic species than the aliphatic components. Modeling interest in dissolution is directed at predicting the concentrations of dissolved components in the water column. Most models in existence do not separate the dissolution component. The solubility of oil components in water varies widely depending on composition.
Solubility decreases very rapidly with increasing size and increasing substitution. In contrast, the solubility of the aliphatic oil components is very low relative to that of aromatic hydrocarbons and is considered to be negligible. The solubility of crude oils and petroleum products was investigated by Shiu et al. (1990) using several methods in two different laboratories and under a variety of conditions. Brookman et al. (1985) also reviewed the solubility of oil and oil components in water.
The rate and extent to which an oil dissolves depends upon its composition, spreading, the water temperature, turbulence, the sea condition and degree of dispersion. The heavy components of crude oil are virtually insoluble in sea water whereas lighter compounds, particularly aromatic hydrocarbons such as benzene and toluene, are slightly soluble. However, these compounds are also the most volatile and are lost by evaporation quicker than being soluble.
Figure 5
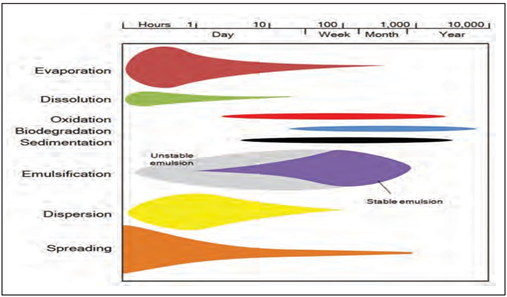
|
Figure 5 A Typical Schematic Representation of Oil Weathering Processes. (ITOPF 2005) |
6)
Dispersion
The rate of dispersion is largely dependent upon the nature of the oil, type of the oil and the sea state, proceeding most rapidly with low viscosity oils in the presence of breaking waves. Waves and turbulence at the sea surface can cause all or part of a slick to break up into droplets of varying sizes which become mixed into the upper layers of the water column. Usually distributed about 5m below the water column. Smaller droplets remain in suspension while the larger ones rise back to the surface where they either coalesce with other droplets to reform a slick or spread out in a very thin film.
For those droplets smaller than about 70μm in diameter, the speed with which they rise towards the surface is balanced by the turbulence of the sea so that they are held in suspension. This dispersed oil mixes into ever greater volumes of sea water, resulting in the rapid and very substantial reduction of the oil concentration. The increased surface area presented by dispersed oil also promotes processes such as biodegradation, dissolution, and sedimentation.
7)
Oxidation
Crude oil is a complex mixture of organic compounds, mostly hydrocarbons. Oxidation alters these mixtures by creating new compounds and by rearranging the distribution pattern of residual compounds, based on their susceptibility to the oxidative process. The ultimate by-products of the oxidative fate of all the organic compounds, given sufficient supply of oxygen and time, is conversion to harmless carbon dioxide and water, as expressed in the following equation:
CH2O + O2 <—> CO2 + H2O
Where CH2O is a symbol for all organic compounds, CO2 is carbondioxide, H2O is water and O2 is oxygen.
In the oxidation of crude oil, hydrocarbons are oxidized to alcohols, ketones, and organic acids. Oxidized products are more water soluble than the hydrocarbon compounds from which they are derived. The order in which hydrocarbons are oxidized depends on a variety of factors, but in general, small molecules up to C20 (molecules with 20 carbon atoms or less) are oxidized before larger ones. Within the same molecular weight range, the order is the aliphatic n-paraffins (n-alkanes) first, followed by branched and cyclic alkanes (naphthalenes) and then the polycyclic aliphatic and aromatic hydrocarbons. Thus, the degree of oxidation can be ascertained on the residue, based on the type and distribution of the residual compounds.
Oxidation of crude oil is mediated by two processes, photooxidation and microbial oxidation, that provide the energy to drive the oxidative reactions. Where crude oil is exposed to sunlight and oxygen in the environment, both photooxidation and aerobic microbial oxidation take place. Where oxygen and sunlight are excluded in anoxic environments, anaerobic microbial oxidation takes place.
13. Photooxidation in Sea Water
Photooxidation is a family of light-catalyzed reactions that oxidize the reduced carbon in petroleum hydrocarbons. These reactions include both direct photoreactions, where the reactant absorbs light energy, to form a less stable intermediate, and indirect photoreactions, where other chemical species in solution absorb light energy. Both produce reactive intermediates (e.g., solvated electrons, hydroxy radicals) that attack the hydrocarbon molecule or transfer energy directly to the reactant hydrocarbon.
The necessary ingredients for photooxidation are radiation and light-absorbing molecules (chromophores). Because few petroleum hydrocarbons absorb sunlight efficiently, most photooxidation occurs via indirect photoreactions. The formation of singlet oxygen from the energy transfer of the triplet excited state of natural organic matter in sea water provides the dominant oxidant for this reaction. Heterogeneous photooxidation, in which reactions occur at the liquid-solid and liquid-liquid interfaces, may also be important. Heterogeneous photolysis of adsorbed species on natural particulate matter may result from direct photochemistry, surface semiconductor redox reactions, or photosensitized reactions on the surfaces of algal cells.
14. Microbial Oxidation
There are generally two biological fates of petroleum in marine systems. Both utilize the same metabolic pathway, respiration, but have different end points. The first process utilizes hydrocarbons as a carbon source to produce energy, while subsequently degrading the long-chained.
The second biological process is primarily a detoxification mechanism in response to exposure to oil whereby an organism metabolizes the hydrocarbons to more water-soluble products that can be excreted from the body.
15. Biodegradation
Biodegradation of hydrocarbons has been considered one of the principal removal mechanisms in the aquatic environment pollutions. Leahy and Colwell (1990), Heider et al. (1999).
Sea water contains a range of marine micro-organisms capable of metabolising oil compounds. They include bacteria, moulds, yeasts, fungi, unicellular algae and protozoa, which can utilise oil as a source of carbon and energy.
There are several energetically favorable metabolic pathways to degrade hydrocarbons that are utilized by different types of microorganisms, including oxidative phosphorylation or respiration (heterotrophic bacteria, fungi, and heterotrophic phytoplankton), nitrate reduction (denitrifiers), and sulfate reduction.
The main factors affecting the rate and extent of biodegradation are the characteristics of the oil, the availability of oxygen and nutrients (principally compounds of nitrogen and phosphorus), suitable humidity, pH, sunlight and temperature. A number of intermediate compounds are produced as the hydrocarbons are broken down but the eventual products of biodegradation are carbon dioxide and water.
16. Sedimentation/Sinking
Dispersed oil droplets can interact with sediment particles and organic matter suspended in the water column so that the droplets become dense enough to sink slowly to the seabed. Shallow coastal areas and the waters of river mouths and estuaries are often laden with suspended solids that can bind with dispersed oil droplets, thereby providing favorable conditions for sedimentation of oily particles. In brackish water, where fresh water from rivers lowers the salinity of sea water and therefore its specific gravity, neutrally buoyant droplets of oil may sink. Oil may also be ingested by planktonic organisms and incorporated into faecal pellets which subsequently drop to the seabed. In rare instances, oil can become entrained with high levels of suspended solids during storm conditions and fall to the seabed. Similarly, wind-blown sand may sometimes be deposited on top of floating oil and cause it to sink.
Most oils have sufficiently low specific gravities to remain afloat unless they interact with and attach to more dense materials. However, some heavy crude oils, most heavy fuel oils and water-in-oil emulsions have specific gravities close to that of sea water and even minimal interaction with sediment can be sufficient to cause sinking. Only a very few residual oils have specific gravities greater than sea water (>1.025), thereby causing them to sink once spilled.
Sedimentation is one of the key long-term processes leading to the accumulation of spilled oil in the marine environment. However, sinking of bulk oil is only rarely observed other than in shallow water, close to shore, primarily because of shoreline interaction.
Sinking is the mechanism by which oil masses that are denser than the receiving water are transported to the bottom. The oil itself may be denser than water, or it may have incorporated enough sediment to become denser than water. Sedimentation is the sorption of oil to suspended sediments that eventually settle out of the water column and accumulate on the seafloor. Conover (1971).
17. Fates of Subsurface Releases
Oil spills sometimes are not released at the surface but further down in the water column. Examples include natural seeps, blowouts during drilling of exploratory wells, pipeline leaks, and shipwrecks. Subsurface releases differ from surface releases in several important ways. First, oil can move substantial distances beneath the surface before it finally floats to the surface. This is especially true in deeper water where currents are strong. Such behavior makes tracking difficult, but more importantly it potentially provides time for the more soluble oil fractions to dissolve. Dissolution is enhanced because of mixing and higher pressure, and other aspects.
Subsurface releases are separated into deepwater and shallow water, corresponding to a break at roughly 200 m. The separation is due to a number of physical and chemical complications that arise in deeper water.
18. Fate of Shallow-Water Releases
Considerable research has been conducted and measurements have been made on subsea shallow releases in the last 25 years. Fannelop and Sjøen (1980). Through these efforts, the so-called integral plume models evolved based on an Eulerian reference frame. Yapa and Zheng (1997) improved the efficiency of the integral plume model using a Lagrangian scheme and incorporated more realistic ambient currents. Arguably their biggest contribution has been in verifying the integral plume model through extensive comparisons with analytical solutions and through laboratory and field experiments.
19. Fate of Deepwater Releases
Substantial efforts to study petroleum releases in deeper water have only recently begun, although there were a few early efforts—most notably studies of deepwater gas hydrate formation by Bishnoi and Maini (1979). Most recent efforts began in 1997 and were triggered by the expansion of hydrocarbon exploration efforts into deeper water. Results are starting to appear: Johansen (2000), Masutani and Adams (2000), Johansen et al. (2001), Yapa et al. (2001), and Adams and Socolofsky (2001). Deepwater releases are much more complicated than those in shallow water.
The majority of the oil in most deepwater releases will rise to the surface although it may take several hours. Theoretical calculations of a light-weight oil suggests that, at most, 10 percent might dissolve under perfect conditions. For diesel and a light medium crude, it observed that it takes about an hour for hydrocarbons to appear at the surface when released from 800 m of water.
The surface slick formed once the oil reaches the surface will be thinner than that seen during a shallow-water release or a surface release. This is due in part to the fractionation of oil droplets that results in a staged arrival of the oil at the surface and in part to diffusion or dispersion of the oil as it rises.
Much if not all of the natural gas (85 percent methane or more) associated with the oil will likely be dissolved in the water column, regardless of whether hydrate forms or not. This is due to the high solubility of methane in sea water at the high pressures and cold temperatures found in deeper water.
Integral plume models like those of Johansen (2000) and Yapa et al. (2001) do appear to capture much of the major features observed in the field including the terminal layer and time to surface.
Despite the fact that surface slicks capture the public attention, there are a substantial percentage of accidental spills that occur beneath the surface, typically from the seafloor. Examples include blowouts of exploration wells, pipeline ruptures, and shipwrecks.
The release of oil beneath the surface introduces a number of complications compared to oil released at the surface. From the standpoint of fate the most important complications are enhanced dissolution in the water column and, perhaps, emulsification. If natural gas is present it will tend to dissolve rapidly during the rise through the water column.
20. Oil Groupings
Oil is grouped into four major groups based on the type of oil, distillation characteristics, viscosity, density, volatility, flash point, pour point, metal content and the asphaltene content.
Table 1
|
Table 1 Oil Classification Based on their °API (American Petroleum Institute Gravity) (ITOPF 2005) |
|||||
|
GROUP
1 OILS |
|
|
|
|
|
|
A:
'API > 45 (Specific gravity < 0.8) |
|||||
|
B:
Pour point ⁰C |
|||||
|
C:
Viscosity 0 at 20 ⁰C : less than 3 CSt |
|||||
|
D:%
boiling below 200 ⁰C: greater than 50% |
|||||
|
E:%
boiling above 370 ⁰C: between 29 and 0% |
|||||
|
Aasgard |
49 |
-28 |
2 @
10⁰C |
58 |
14 |
|
Arabian
Super Light |
51 |
-39 |
2 @
20⁰C |
||
|
Cossack |
48 |
-18 |
2 @
20⁰C |
51 |
18 |
|
Curlew |
47 |
-13 |
2 @
20⁰C |
57 |
17 |
|
F3
Condensate |
54 |
<-63 |
1 @
10⁰C |
81 |
0 |
|
Gippsland |
52 |
-13 |
1.5
@ 20⁰C |
63 |
8 |
|
Hidra |
52 |
-62 |
2.5
@ 10 ⁰C |
60 |
11 |
|
Terengganu
Condensate |
73 |
-36 |
0.5
@ 15⁰C |
>95 |
0 |
|
Wollybutt |
49 |
-53 |
2
@20 ⁰C |
55 |
4 |
|
Gasoline |
58 |
0.5
@ 15⁰C |
100 |
0 |
|
|
Kerosene |
45 |
-55 |
2
@15⁰C |
50 |
0 |
|
Naptha |
55 |
100 |
0 |
||
Table 2
|
Table 2 Oil Classification Based on their °API (American Petroleum Institute Gravity) (ITOPF 2005) |
||||
|
GROUP
2 OILS |
||||
|
A:
'API 35-45 (Specific gravity 0.8-0.85) |
||||
|
B:
Pour point ⁰C |
||||
|
C:Viscosity
@ 10- 20 ⁰C : Between 4 CSt and
semi-solid |
||||
|
D:%
boiling below 200 ⁰C : between 20 and 50% |
||||
|
E:%
boiling above 370 ⁰C : between
15 and 50% |
||||
|
Low
pour point <6⁰C |
||||
|
A |
B |
C |
D |
|
|
Arabian
Extra Light |
38 |
-30 |
3
@15 ⁰C |
26 |
|
Azeri |
37 |
-3 |
29 |
|
|
Brent |
38 |
-3 |
7 @
10⁰C |
37 |
|
Draugen |
40 |
-15 |
4 @
20 ⁰C |
37 |
|
Dukhan |
41 |
-49 |
9
@15 ⁰C |
36 |
|
Liverpool
Bay |
45 |
-21 |
4 @
20 ⁰C |
42 |
|
Sokol(Sakhalin) |
37 |
-27 |
4 @
20 ⁰C |
45 |
|
Rio
Negro |
35 |
-5 |
23 @
10 ⁰C |
29 |
|
Umm
Shaif |
37 |
-24 |
10 @
10 ⁰C |
34 |
|
Zakum |
40 |
-24 |
6
@10⁰C |
36 |
|
Marine
Gas Oil (MGO) |
37 |
-3 |
5 @
15 ⁰C |
|
|
High
Pour Point >5⁰C |
||||
|
Amna |
36 |
19 |
Semi-solid |
25 |
|
Beatrice |
38 |
18 |
32
@15 ⁰C |
25 |
|
Bintulu |
37 |
semi-solid |
24 |
34 |
|
Escravos |
34 |
10 |
9
@15 ⁰C |
35 |
|
Sarir |
38 |
24 |
SemiSolid |
24 |
|
Statfjord |
6 |
38 |
||
Table 3
|
Table 3 Oil Classification Based on their °API (American Petroleum Institute Gravity) (ITOPF 2005) |
|||||
|
GROUP
3 OILS |
|||||
|
A:
'API 17.5 -35 (Specific gravity
0.8-0.95) |
|||||
|
B:
Pour point ⁰C |
|||||
|
C:Viscosity
@ 10 - 20 ⁰C : less than 8 CSt
and Semi Solid |
|||||
|
D:%
boiling below 200 ⁰C : between 10 and 35% |
|||||
|
E:%
boiling above 370 ⁰C : between
30 and 65% |
|||||
|
Alaska
North Slope |
28 |
-18 |
32 @
15 ⁰C |
32 |
41 |
|
Arabian
Heavy |
28 |
-40 |
55 @
15 ⁰C |
21 |
56 |
|
Arabian
Medium |
30 |
-21 |
25 @
15 ⁰C |
22 |
51 |
|
Arabian
Light |
33 |
-40 |
14 @
15 ⁰C |
25 |
45 |
|
Bonny
Light |
35 |
-11 |
25 @
15 ⁰C |
26 |
30 |
|
Iranian
Heavy |
31 |
-36 |
25 @
15 ⁰C |
24 |
48 |
|
Iranian
Light |
34 |
-32 |
15
@ 15 ⁰C |
26 |
43 |
|
Khafji |
28 |
-57 |
80 @
15 ⁰C |
21 |
55 |
|
Sirri |
33 |
-12 |
18 @
10 ⁰C |
32 |
38 |
|
Thunder
Horse |
35 |
-27 |
10 @
10 ⁰C |
32 |
39 |
|
Tia
Juana Light |
32 |
-42 |
500
@ 15 ⁰C |
24 |
45 |
|
Troll |
33 |
-9 |
14 @
10 ⁰C |
24 |
35 |
|
IFO
180 |
18-20 |
Oct-30 |
1,500
-3,000 @ 15⁰C |
- |
|
|
High
Pour point > 5⁰C |
|||||
|
Cabinda |
33 |
12 |
Semi-solid |
18 |
56 |
|
coco |
32 |
21 |
Semi-solid |
21 |
46 |
|
Gambia |
31 |
23 |
Semi-solid |
11 |
54 |
|
Mandji |
30 |
9 |
70 @
15 ⁰C |
21 |
53 |
|
Minas |
35 |
18 |
semi-solid |
15 |
58 |
Table 4
|
Table 4 Oil Classification Based on their °API (American Petroleum Institute Gravity) (ITOPF 2005) |
|||||
|
GROUP
4 OILS |
|||||
|
A:
'API > 17.5 (Specific gravity <
0.95) |
|||||
|
B:
Pour point >30 ⁰C |
|||||
|
C:Viscosity
@ 10 - 20 ⁰C : between 1500 CSt
and semi solid |
|||||
|
D:%
boiling below 200 ⁰C : less than 25% |
|||||
|
E:%
boiling above 370 ⁰C : greater
than 30% |
|||||
|
A |
B |
C |
D |
E |
|
|
Bachaquero
17 |
16 |
-29 |
5,000
@ 15 ⁰C |
10 |
60 |
|
Boscan |
10 |
15 |
Semi-Solid |
4 |
80 |
|
Cinta |
33 |
43 |
Semi-Solid |
10 |
54 |
|
Handil |
33 |
35 |
Semi-Solid |
23 |
33 |
|
Merey |
17 |
-21 |
7,000
@ 15 ⁰C |
7 |
70 |
|
Nile
Blend |
34 |
33 |
Semi-Solid |
13 |
59 |
|
Pilon |
14 |
-3 |
Semi-Solid |
2 |
92 |
|
Shengli |
24 |
21 |
Semi-Solid |
9 |
70 |
|
Taching |
31 |
35 |
Semi-Solid |
12 |
49 |
|
Tia
Juana Pesado |
12 |
-1 |
Semi-Solid |
3 |
78 |
|
Widuri |
33 |
46 |
Semi-Solid |
7 |
70 |
|
IFO
380 |
Nov-15 |
Oct-30 |
5,000-30,000
@ 15 ⁰C |
||
21. Contingency Planning
for Clean-up
The tendency of oil to spread and fragment rapidly, especially in rough sea conditions, will always place constraints on any response strategy and should not be underestimated. For instance, ship-borne oil recovery systems, with swath widths of typically only a few metres, will be unable to encounter any significant quantities of oil once it has spread and scattered over several kilometres. In the case of low viscosity oils, this can happen in just a few hours. This is one of the main reasons that oil recovery operations at sea rarely achieve the removal of more than a fraction of a large slick. Therefore, the need for a proper and effective contingency planning.
Figure 6
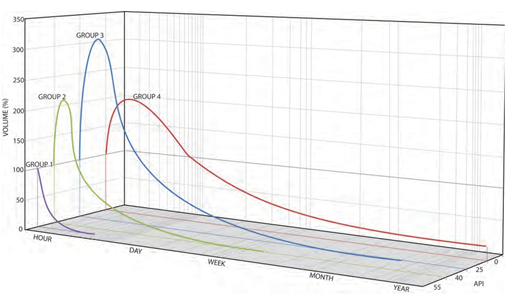
|
Figure 6 The Persistency and Behavior of Different Groups of Oil with Time. (ITOPF2005) |
The movement of slicks, volume of spill and the changing nature of the oil through weathering can determine whether any response, beyond monitoring the dissipation of the slick, is necessary. Where an active response is called for, the weathering processes will require the suitability of selected clean- up strategies to be re-evaluated and modified as the response progresses and conditions change. For example, dispersants applied at sea reduce in efficiency as the oil spreads and as oil viscosity increases. Depending on the characteristics of the particular oil, many dispersants become significantly less effective as the viscosity approaches 10,000 cSt and most cease to work at all when the viscosity rises much above this value. Oil viscosity can increase very quickly, meaning the window of opportunity available for using dispersants can be very short. Consequently, dispersant application should be regularly monitored, and spraying operations terminated if ineffective and other options explored and considered.
Similarly, if mechanical recovery systems are deployed, the type of skimmers and pumps used may need to be changed as the oil weathers, its viscosity rises and emulsions form. For example, oleophilic (oil attracting) disc skimmers rely on oil adhering to the disc for recovery. However, an emulsion acts as a ‘shear-thinning’ fluid such that when a twisting movement is applied, for example by a spinning disc, the water droplets in the emulsion align all in one direction, reducing viscosity and causing the emulsion to be sliced through rather than adhering to the disc. The same effect occurs with centrifugal pumps, where the pump impellor may spin without efficient movement of the emulsion through the pump. For this reason, positive displacement pumps are recommended for the transfer of emulsions.
22. Conclusion
The behavior and fate of crude oil and refined products in the marine environment are controlled by many different processes that vary considerably in space and time. Physical, chemical, and biological processes all interact to (1) alter oil introduced into the oceans; (2) transport the resulting degradation (weathering) products away from the source; and (3) incorporate the residual substances into compartments of the earth's surface system. These compartments involve dissolution in the hydrosphere, deposition in the lithosphere, volatilization into the atmosphere, and ingestion by organisms in the biosphere. Physical processes degrading oil include evaporation, emulsification, and dissolution, whereas chemical processes focus on photooxidation and biological processes emphasize microbial oxidation.
The transportation of oil in the marine environment occurs in two directions, horizontal and vertical. Horizontal transport involves spreading and surface advection, leading in some instances to shoreline stranding and tarball formation. Transport in the vertical direction includes dispersion, entrainment, Langmuir circulation, sinking, overwashing, and sedimentation.
Oil entering the marine environment comes from natural sources (oil seeps) and from sources over which humankind has some control (oil spills, urban runoff, pollution resulting from oil transportation and production, and oil usage in vehicles, including boats). The ultimate fates of oil in the sea depend on the amount and rate of discharge, composition, source, and environmental setting and persistency.
The effect of petroleum hydrocarbon is not directly related to the volume released. But a complex function of the rate of release, the nature of the released hydrocarbon, the type of oil, properties and characteristics of the released oil and the local physical and biological ecosystem.
A better understanding of the behavioral changes of the released oil in the environment is directly proportional to the effective response management, better strategy choice and the overall clean-up success factor which is determined by the volume of spill, type of oil, time of spill, environmental condition at the time of spill and the ocean dynamics.
Prompt response to activating the contingency plan arrangements will always protect the environment and ensure sustainability.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Adams, S. A., & Socolofsky, S.
A. (2001). Estimating Hydrate Formation and
Decomposition of Gaseous Release.
Bautista, H., & Rahman, K.
M. M. (2016). Effects of Crude Oil Pollution in the
Tropical Rainforest Biodiversity of Ecuadorian Amazon Region. Journal of
Biodiversity and Environmental Sciences, 8(2), 249–254.
Bishnoi, P. R., & Maini, B.
B. (1979). A Kinetic Study of Methane Hydrate
Formation.
Conover, W. J. (1971). Practical Nonparametric Statistics (pp. 295–301, 309–314). John Wiley & Sons.
Fannelop, T. K., & Sjøen, K. (1980). Hydrodynamics of Underwater Blowouts. https://doi.org/10.2514/6.1980-219
Fiest, D. L., Boehm, P. D., Rigler, M. W., & Patton, J. S. (1981). Ixtoc 1 Oil Spill: Flaking of Surface Mousse in the Gulf of Mexico. Nature, 290(5803), 235–238. https://doi.org/10.1038/290235a0
Fingas, M., Hollebone, B.,
Fieldhouse, B., & Wang, Z. (1996). The
Evaporation of Oil Spills.
Heider, J., Spormann, A. M.,
Beller, H. R., & Widdel, F. (1998). Anaerobic
Biodegradation of Hydrocarbons.
International Maritime Organization. (2005). [IMO Guidelines or Publication Title if Available].
Johansen, S. (2000). Modelling of Cointegration in the Vector Autoregressive Model. Economic Modelling, 17(3), 359–373. https://doi.org/10.1016/S0264-9993(99)00043-7
Leahy, J. G., & Colwell, R. R. (1990). Microbial Degradation of Hydrocarbons. Microbiological Reviews, 54(3), 305–315. https://doi.org/10.1128/mr.54.3.305-315.1990
Masutani, S. M., & Adams,
E. E. (2000). Experimental Study of Multi-Phase
Plumes with Application to Deep Ocean Oil Spills.
Shiu, W. Y., Bobra, A. M., & Mackay, D. (1990). Effects of the Water-Soluble Fraction of Crude Oil.
Stiver, W., & Mackay, D. (1984). Evaporation Rate of Spills of Hydrocarbons and Petroleum. Environmental Science & Technology, 18(11), 834–840. https://doi.org/10.1021/es00129a006
Yapa, P. D., & Zheng, L. (1997). A Model for Simulating Deepwater Oil and Gas Blowouts.
Yapa, P. D., Zheng, L., & Chen, F. (2001). A Model for Deepwater Oil/Gas Blowouts. Marine Pollution Bulletin, 43(7–12), 234–241. https://doi.org/10.1016/S0025-326X(01)00086-8
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.