
DETERMINATION OF THERMAL ENERGY AND WASTES GENERATION RESULTING FROM INCINERATION OF ONE TON OF CRUDE OIL CONTAMINATED SOILS OF QURNA OIL FIELDS IN BASRA, IRAQ
Ahmed H. Hadi 1![]() , Ahmed Hatif Ahmed 2
, Ahmed Hatif Ahmed 2![]() , Basim. A. Hussain 3
, Basim. A. Hussain 3![]() , Abdullah F. Abd AL Razak 4
, Abdullah F. Abd AL Razak 4![]()
1 Ph.D.
Mechanical Engineering Treatment and Disposal of Hazardous Waste Department,
Ministry of Science and Technology, Baghdad, Iraq
2 Ph.D.
Chemistry Science Treatment and disposal of Hazardous Waste Department,
Ministry of Science and Technology, Baghdad, Iraq
3 Ph.D.
Environmental Engineering Treatment and disposal of Hazardous Waste Department,
Ministry of Science and Technology. Baghdad, Iraq
4 B.Sc.
Energy Engineering, Baghdad, Iraq
|
|
ABSTRACT |
||
|
A
theoretical study is presented to calculate the thermal energy and wastes
generated from complete combustion of (1 ton/h) of Qurna crude oil
contaminated soil in Basra city south of Iraq. Practical values which are
valid in the literature are taken for the constituents of the soil and the
crude oil. A multi chamber incinerator with capacity of (1 ton/h) is designed
to incinerate the crude oil contaminated soils using diesel fuel to reach to
(1200˚C) burning temperature. The resulted thermal energy was found to
be (5.59 MW) which is sufficient to evaporate (9918.449 liter/h) of
industrial wasted water using many of Shore VAP water evaporators systems.
The resulting hazardous flue gases and wastes obtained were (CO2=1773.46
kg/h, SO2 = 13.58kg/h, H2SO4 =0.643125kg/h
(350 ml/h)) using excess air ratios of 150% for solid wastes burning and 20%
for diesel fuel burning. |
|||
|
Received 16 April 2024 Accepted 19 May 2024 Published 21 June 2024 Corresponding Author Ahmed H.
Hadi, ahmed_nu9@yahoo.com DOI 10.29121/IJOEST.v8.i3.2024.601 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Contaminated
Soil with Crude Oil, Treatment by Incineration Method, Multi Stage
Incinerator Design, Thermal Energy, Hazardous Sulfuric Content with Flue
Gases |
|||
1. INTRODUCTION
The contamination process of soil with crude oil occurs in the case of drilling process to get the crude oil or in the process of crude oil transportation Torero et al. (2003). The effect of crude oil on the soil and environment is dangerous as it causes many changes in the chemical and agricultural properties of the soil Wu et al. (2000) studied the effect of crude oil contamination on the chemical properties of soil in Qurna and Medynah cities in Basra government in south of Iraq. Chemical analyses for the contaminated soil from twenty different locations near the petroleum industry companies were made. The chemical properties that were investigated included organic material, Calcium Carbonate (lime), Calcium Sulphate (gypsum), degree of soil interaction (PH) and soil salinity (electrical conductivity) concentrations during two seasons in the year (Summer and Winter).
There are many different technologies applied to treat contaminated soil with crude oil such as chemical, physical, biological and thermal method (incineration). Almutairi (2016) used a physical process to wash the Kuwait contaminated soil with crude oil from resulting from the 2nd Gulf war actions using sea water and adding suitable detergents and centrifuge and vibrations with ultra-sonic waves as a new successful method to split soil from crude oil. This technology yet showed that large quantities of wasted water were produced in which it need to be treated. The biological method to treat the contaminated soil with crude oil using a certain kind of bacteria is more environmentally friendly and it is the best method but it is very slow (needs to many months) and it is not suitable for very hot climates (see Almutairi (2016)) such as that existing in the city of Basra which reaches to more than 50˚C in summer and it's temperature in January is above 20˚C (see Al-Musawi & Muhsin (2015) and Deeri & Al-Kaabi (2014)).
John & Swamy (2011) and Ganguly et al. (2017) designed a multi chamber incinerator to treat the medical wastes of many hospitals in India. They presented a detailed procedure for material and heat balance which is necessary to design a suitable multi chamber incinerator. The same procedure is considered in the current research.
An alternative method for crude oil contamination treatment is the incineration process. This method is quick, economical but with dangerous flue gases that need to be treated by modern off gas systems which are still in the laboratory development stages to make the incineration process more environmental friendly by converting the dangerous flue gases such as CO2, SO2 and H2SO4 vapor to useful materials and treat hot flue gases at (700 ˚C) while the current off gas systems treat flue gases at (40 ˚C) by cooling flue gases using a suitable heat exchanger (see Liemans & Thomas (2013), Al-Mamoori et al. (2017) and Al-Mamoori et al. (2020) and Ashadi et al. (2020)). The treatment of hazardous flue gases at high temperatures at industrial scale can give a chance to convert the thermal energy of hot flue gases from burning of hazardous wastes to electrical energy.
Hadi et al. (2023) studied theoretically and experimentally the utilization of hazardous wastes flue gases to produce electrical energy using a prototype small system with converted steam engine and a water tube boiler with capacity of (120 kg steam/hr) and their results showed that for small quantities of wastes it is more economical to utilize wasted thermal energy generated from the incineration process of hazardous wastes in evaporation of wasted industrial water rather than producing of electrical energy.
The aim of the present research is to design of a multi
chamber incinerator with burning capacity of (1 ton/h) and determine the
quantity of thermal energy released from burning (1 ton/h) of the contaminated
soil with crude oil with ratio of (2:1) obtained from Qurna city in Basra
government south of Iraq, this is a very important step towards using of wasted
thermal energy in evaporation of wasted industrial water in a future research
(under preparation). The comparison between many methods (Thermal, physical,
chemical and biological) to treat the contaminated soil with crude oil and
choice the best method for Iraqi environment is considered in a future research
(under preparation).
2. THEORETICAL ANALYSIS
2.1. ASSUMPTIONS
1)
Complete
combustion process.
2)
Air
contains 23% O2 and 77% N2 by weight.
3)
The
organic material in soil contains (0.8 C+0.2 H).
4)
The Iraqi
crude oil in Basra contains only C, H and S.
5)
The
treatment process is done in July where average ambient temperature between (6
A.M. and 4 P.M.) is 45˚C.
6)
The volume
of any (1 kg/mole) ideal gas is equal to 22.4m3 at (0˚C and 1 atm).
7)
The used
fuel in burning process is diesel with chemical composition (C12H23)
and burning temperature is 1200˚C.
2.2. CRUDE OIL CONSTITUTENTS
The Iraqi crude oil
in Basra has relatively high Sulphur contains which is 2.1% by weight that
produces acidic gases after burning like SOx and H2SO4
vapor (see IPCC. (2001)), the crude oil compositions are given in the
following equation:
%Wcrude-oil=0.841C+0.138H+0.021S
(1)
2.3. SOIL CONSTITUTENTS IN QURNA CITY
Depending on Al-Musawi & Mustafa (2019). experimental measurements, in the present
research the data of Al-Jary site is taken to be the basis of this research
calculations because Al-Jary site is located inside West Qurna petroleum
factory and north to the 8th gas station and its soil constituents are given in
the following equations:
% Wsoil-summer = 0.03683 Ca CO3 + 0.01756 Ca SO4 +
0.0067 O.M. + 0.93891 others
(2)
% Wsoil-winter = 0.03699 Ca CO3 + 0.01544 Ca SO4 +
0.00692 O.M. + 0.94065 others
(3)
The ratio of soil to
crude oil was taken as (2:1) as in Almutairi (2016), to get weight fraction of 1 kg contaminated
soil with crude oil in summer multiplying equation 1 by (1/3) added to equation
2 multiplied by (2/3) and by substituting (O.M. = 0.8 C+0.2 H) and rearrange
the resulted equation gives:
% W contaminated soil (1 kg) = 0.283906336 C + 0.046893334 H + 0.007 S +
0.0245533 Ca CO3 + 0.01170667 Ca SO4 + 0.62594
others (4)
The weight fraction
of 1 ton of contaminated soil with crude oil is given in the following
equation:
% W contaminated
soil (1 ton) = 283.906336 C + 46.893334 H + 7 S + 24.5533 Ca CO3 +
11.70667 Ca SO4 + 625.94 others
(5)
2.4. MATERIAL BALANCE
2.4.1. STOICHIOMETRIC CALCULATIONS
In equation (5) when
the components are burned under (1200˚C) the terms of C, H and S react
with O2 while the terms of Ca SO3 and others are
unchanged, the Ca CO3 under (900˚C) converts to Ca O and CO2as
in the following equation:
 (6)
(6)
The Carbon reacts with oxygen as in the
following equation:
 (7)
(7)
The Hydrogen reacts with oxygen as in the
following equation:
 (8)
(8)
The Sulphur reacts
with oxygen as in the following equation:
 (9)
(9)
The total
stoichiometric quantities for O2, CO2 and H2O
from equations (6, 7, 8 and 9) are (1139.32487 kg, 1051.887986112 kg and 422.04
kg) respectively.
2.4.2. EXCESS AIR AND MOISTURE CALCULATIONS
The excess air to
burn wastes in a multi chamber incinerator is 150% (John & Swamy (2011)). Thus, the excess O2 is calculated as
follows:
O2 (150% excess) = 2.5 * O2
(stoichiometric) = 2848.312175 kg
Air required = O2 / 0.23 =
12383.966 kg
N2 = air required – O2 =
9535.6538 kg
The moisture in the
combustion air can be determined from the following equations (see Ganapathy (2015)) considering the average ambient temperature
and relative humidity in July in Basra city are 45˚C and 43.2 %
respectively (Deeri & Al-Kaabi (2014) and Al-Musawi
& Muhsin (2015)):
![]() (10)
(10)
Where 1(kg/cm2.a) =
1 kpa/98.0665
Pw=relative humidity*P(kg/cm2)
(11)
From thermodynamics
tables Psat (45˚C) = 9.582 kpa = 0.097709 kg/cm2, Pw = 0.0422103, Moisture
(air) = 0.0265 (kg H2O/ kg air) and the quantity of moisture in air
combustion is (0.0265 * 12383.966 = 328.175 kg H2O).
2.4.3. H2SO4 FORMATION CALCULATIONS
From equation (9)
the produced gas of SO2 (1-3) % from it reacts with O2
and converts to SO3 (Ganapathy (2015)) in which it reacts with water vapor to form
Sulfuric acid vapor that condenses under 150˚C and causes corrosion in the
heat exchanger equipment (Ganapathy (1989)). The equations of H2SO4
formation are as follow:
 (12)
(12)
The SO3
gas reacts with water vapor to form Sulfuric acid vapor as in the following
equation (assumed all SO3 converts to H2SO4):
 (13)
(13)
The volume of
Sulfuric acid after completely condensation at 150 ˚C can be calculated by
substituting its mass over its density (1840 kg/m3) to get nearly
(350 ml).
The final mass of SO2
is (14 kg – 0.42 kg = 13.58 kg), the final mass of O2 in flue gases
is (2848.312175 kg – O2 stoichiometric (1139.32487 kg) – O2
consumed in Sulfuric acid formation (0.105 kg)= 1708.882305 kg).The final mass
of H2O vapor in flue gases is (422.04 kg (eq. 8) + 328.175 kg
(moisture) – 0.118125 kg consumed in Sulfuric acid formation = 750.096875 kg).
2.5. HEAT BALANCE
2.5.1. TOTAL HEAT INPUT FROM WASTES Qi
The high heating
value (H.H.V.) emitted from burning 1 ton of contaminated soil with crude oil
where its constituents are listed in equation (5) can be evaluated from Dulong
equation as follow (see Abd-Alrazak (2008)):
H.H.V.=33960 C+14421
(H2-![]() )+9400 S
(14)
)+9400 S
(14)
The total input heat
(Qi) can be evaluated as listed in table (1) depending on equation (5).
Table
1
|
Table 1 Computation of Input Heat |
||||
|
|
Component |
Mass
(kg/h) |
H.H.V.
(KJ/kg) |
Heat
(MJ/h) |
|
1 |
C |
283.906336 |
33960 |
9641.46 |
|
2 |
H |
46.893334 |
14421 |
676.24877 |
|
3 |
S |
7 |
9400 |
65.8 |
|
4 |
Ca
SO4 |
11.70667 |
0 |
0 |
|
5 |
CaCO3 |
24.5533 |
0 |
0 |
|
6 |
Others |
625.94 |
0 |
0 |
|
|
|
1
ton/h |
|
10383.50877
MJ/h |
2.5.2. TOTAL HEAT OUT BASED ON EQUILIBRIUM TEMPERATURE OF 1200˚C
The output heat Qo
represents the sum of radiation loss, heat to ash, heat to dry combustion
products and heat to moisture in which they can be evaluated as follow:
1- Radiation loss =
5% of input heat = 0.05* Qi = 519.175 MJ/h.
2-Heat to ash = mash
* cpash * (1200-Tamb) (15)
Where mash = m (Ca
O) + m (CaSO4) + m (others) = 651.396518 kg/h
and cpash = 0.381
kJ/kg.˚C (see John & Swamy (2011)) and Tamb = 45˚C, substitute in equation
(15) to get heat to ash = 286.6503 MJ/h.
3- Heat to dry
combustion products (N2, O2 residual, CO2, SO2
and H2SO4)
Qdp =mdp * cpdp * (1200-Tamb)
(16)
Where mdp = m N2
+ m O2 residual + m CO2 + m SO2 + m H2SO4
= 12310.647255 kg/h, cpdp = 1.086 kJ/kg.˚C (see John & Swamy (2011)) and Tamb = 45˚C, substitute in equation
(16) to get Qdp = 15441.6141713 MJ/h.
4- Heat to moisture
= mmoisture*cpmoisture*(1200-45) + mmoisture * hfg
(17)
= 750.096875
*2.347*1155+750.096875*2460.3
= 3878.8147
MJ/h.
From equations above
Qo = 20126.2541713 MJ/h = 5.59 MW.
2.5.3. NET HEAT AND REQUIRED AUXILIARY FUEL
Qnet = Qi – Qo=
-9742.7454013 MJ/h = mdiesel* H.H.V. (diesel)
The negative sign
means need to add auxiliary fuel, there is 5% heat radiation loss from burning
fuel, H.H.V. for diesel is 44.8 MJ/kg and density of diesel is 0.845 kg/liter.
The mass of diesel can be computed as follow:
mdiesel =
1.05*9742.7454013/44.8 = 228.3456 kg/h
Vdiesel =
228.3456/0.845 = 270.23 liter/h.
2.5.4. PRODUCTS OF COMBUSTION FROM AUXILIARY FUEL
Considering the chemical formula for diesel as (C12H23) in which it burns according to the following equation:
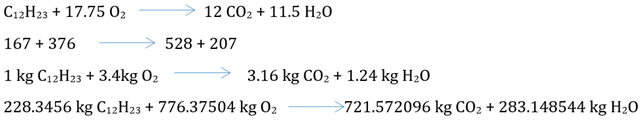
In burning fuel 20%
excess air can be taken and the required quantities can be determined as
follow:
O2
(excess 20%) fuel = 1.2 *776.37504 kg = 931.65 kg, in which 776.37504 kg is
reacted with fuel and 155.275 kg exit as residual O2 with flue
gases.
Air excess (fuel) =
O2 / 0.23 = 4050.6523 kg and N2 = 3119 kg and air
moisture = 4050.6523 * 0.02625 = 106.33 kg which is added with 283.148544 kg to
get the total mass of moisture of the fuel. The final fuel burning equation
with 20% excess air is as follow:
228.3456 kg/h diesel + 3119 kg/h N2 + 931.65 kg/h O2
+ 106.33 kg/h moisture
3119 kg/h N2 + 155.275 kg/h O2 + 721.572096 kg/h
CO2 + 389.478544 kg/h H2O
(19)
2.5.5. THE FINAL EQUATION FOR REACTANTS AND PRODUCTS
![]() 1 ton/h contaminated soil with crude oil +
228.3456 kg/h diesel (270.23 liter /h) + 3779.962175 kg/h O2 +
12654.653825 kg/h N2 + 434.505 kg/h H2O (air moisture)
1200 ˚C
1 ton/h contaminated soil with crude oil +
228.3456 kg/h diesel (270.23 liter /h) + 3779.962175 kg/h O2 +
12654.653825 kg/h N2 + 434.505 kg/h H2O (air moisture)
1200 ˚C
12654.653825 kg/h N2
+ 1864.157313 kg/h O2+ 1773.46 kg/h CO2 + 1139.575419
kg/h H2O + 651.396518 kg/h ash as{11.70667 kg/h Ca SO4 +
13.749848 kg/h Ca O+ 625.94 kg/h others} + 13.58 kg/h SO2 + 0.643125
kg/h H2 SO4 (350 ml/h) + Qo (5.59 MW) (20)
The total mass input
from reactants in equation (20) equals to(18097.4666 kg/h) and the total mass
output from products in equation (20) equals to(18097.4662 kg/h) which equal to
the sum of (651.396518 kg/h ash) and (17446.069682 kg/h flue gases). The flue
gases total mass is the sum of (16306.494263 kg/h dry products) and total
moisture of (1139.575419 kg/h). The weight fraction for flue gases can be given
in the following equation:
% Wflue-gases = 0.725358 N2 + 0.106852 O2 +
0.1016538 CO2 + 0.06531989 H2O + 0.0007783988 SO2
+ 0.0000368636 H2 SO4
(21)
The volume fraction
of flue gases can be found by computing the volume of each gas (using ideal gas
law) and the total volume of flue gases, the volumetric flow rate of each gas
can be determined as in the following equation:
Vgas = mgas *![]() (22)
(22)
The molecular weight
of flue gases (N2, O2, CO2, H2O, SO2
and H2SO4) are (28, 32, 44, 18, 64 and 98) respectively
and the volume of each gas are (15.17322, 1.95577188, 1.35317616, 2.1254759,
0.0071236966 and 0.0002203205) m3/s respectively and the total
volume of flue gases equals to 20.6149879 m3/s. The volume fraction
for flue gases can be given in the following equation:
% Vflue-gases = 0.736028 N2 + 0.094871 O2 +
0.06564 CO2 + 0.103103 H2O + 0.0003455591 SO2
+ 0.0000106873 H2SO4 (23)
2.5.6. DESIGN OF MULTI CHAMBER INCINERATOR
The multi chamber
incinerator contains primary and secondary chambers, to design the primary
chamber for a multi chamber incinerator of burning capacity of (1 ton/h).
Consider the volume of contaminated soil with crude oil of mass 1 ton is nearly
equal to (1 m3) and the volume of heap is (5 m3) as
follow:
Vprimary chamber = L
* B * H= 5 m3
Assuming a suitable
depth of 2.2 m (H) and assume (L/B = 1.5/1) then:
Area = volume /
depth = 5/2.2 = 2.3 m2 = L*B = 2.3 B2 therefore:
B = 1.238 m and L=
1.857 m.
Vprimary chamber = 1.857
m *1.238 m* 2.2 m = 5 m3
(24)
The volume of the
secondary chamber of the multi chamber incinerator that satisfy (1 second)
resident time for flue gases at (1200 ˚C) can be estimated from equation
(22) for dry products (molecular weight for air = 29) and moisture (molecular
weight = 18) then:
Vsecondary chamber = Vdp + Vmoisture = 18.877 + 2.125 = 21.002 m3/s.
This procedure is
the same as that used by John & Swamy (2011), they assumed that dry products have the properties
of air while if dry products considered uniquely the volume of secondary
chamber is equal to the total volume of flue gases (20.6149879 m3/s)
that used to compute volume fraction of flue gases in equation (23) which is
more accurate value than (21.002 m3/s).
3. RESULTS AND DISCUSSION
The results obtained from the current research are valid in equations (20, 21, 23 and 24) where the thermal energy, weight and volume fractions of the flue gases, mass and volume rate of diesel fuel, resulted ash components and dimensions of the multi chamber incinerator are given.
3.1. THERMAL ENERGY
From equation (20) the resulted thermal energy is (5.59 MW), as wasted heat which is dissipated as radiation loss and in ash and as thermal content in flue gases (in dry products and moisture), the useful thermal energy is carried with flue gases, by cooling the flue gases using forced convection (in a suitable heat exchanger to ambient temperature) and condensation of moisture at (100˚C and 1 atm) but at this temperature the sulfuric acid vapor is condensed at 150˚C and its volume of (350 ml) causes corrosion at the cold parts from the heat exchanger at the time between cooling the flue gases from (150˚C to 100˚C) then the moisture of (1139.575419 kg/h) begins to be condensed and mixed with sulfuric acid condensed liquid.
Cooling flue gases to ambient temperature is firstly necessary to treat dangerous flue gases using suitable designed industrial off gas system and secondly it is best to get more thermal energy but the problem of sulfuric acid condensation and its corrosion effects preventing to cool flue gases under 200˚C. Modern researches treat flue gases at 700˚C successfully but they still in laboratory scale and may be they can reach industrial scale about 2030, at that time it can be used more wasted thermal energy by cooling flue gases to ambient temperature.
3.2. RESULTED WASTES
The resulted wastes from incineration process of contaminated soil with crude oil are ash and flue gases as in equation (20). The ash has mass of (651.396518 kg/h) and its volume is less than the primary volume of the contaminated soil by nearly (1/3) where it can be treated and disposed after it cooled to ambient temperature.
The mass of flue gases is (17446.069682 kg/h) and the dangerous components are (1773.46 kg/h CO2, 13.58 kg/h SO2 and 0.643125 kg/h H2SO4). The mass of flue gases is big because of big excess air necessary to burn solid wastes (150%) compared with excess air necessary for burning liquid fuel (20%), the weight and volume fractions of flue gases are valid in equations (21 and 23) respectively, the quantity of N2 represents more than 70% in weight and volume fraction and that causes firstly need of more fuel to reach (1200˚C) where (270.23 liter/h) of diesel fuel is needed and secondly large incinerator dimensions is needed because of the large volume of resulted flue gases (21.002 m3/s). To solve this problem, modern researches use oxy-combustion method to prevent N2 gas and in the same time this method is economic in fuel consumption and leads to less CO2 emissions because less fuel is needed.
4. CONCLUSIONS
1) The design of multi chamber incinerator, the primary chamber dimensions are (1.857 m * 1.238 m * 2.2 m = 5 m3), the secondary chamber volume is (21.002 m3/s). A diesel fuel (C12H23) is used with volumetric rate of (270.23 liter/h).
2) Treatment of soil with incineration method is quick and economical yet it produces hazardous flue gases in which completely treatment at high temperatures (700˚C) is still in laboratory research development.
3) The resulted thermal energy is (5.59 MW) which is sufficient to evaporate (9918.449 liter/h) of industrial wasted water using many of Shore VAP water evaporators systems.
4) The incineration products are 651.396518 kg/h ash and 17446.069682 kg/h flue gases.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors and researchers of this study would like to thank the hazardous waste treatment and disposal directory in Ministry of Science and Technology in Iraq for their help and support to complete this research.
REFERENCES
Abd-Alrazak, W. H. (2008). Studying the Effects of Using Different Kinds of Fuel on the Efficiency of Steam Boiler [In Arabic]. Technical Institute, Al-Dor, Iraq.
Al-Mamoori, A., Alghamdi, T., Rownaghi, A. A., & Rezaei, F. (2020). Enhancing the Ethylene Yield Over Hybrid Absorbent Catalyst Materials in CO2-Assisted Oxidative Dehydrogenation of Ethane by Tuning Catalyst Support Properties. Journal of Energy and Fuels. https://doi.org/10.1021/acs.energyfuels.0c02750
Al-Mamoori, A., Krishnamurthy, A., Rownaghi, A. A., & Rezaei, F. (2017). Carbon Capture and Utilization Update. Energy Technology, 5, 1-17. https://doi.org/10.1002/ente.201600747
Al-Musawi, A. S., & Muhsin, B. N.
(2015). The Spatial Relationship Between Relative
Humidity and Phenomena Dust in Iraq [In Arabic]. Journal of Geographic
Researches, University of Kufa, Iraq.
Al-Musawi, N. A., & Mustafa, S. W. (2019). Impact of Oil Pollution on Chemical Properties for Soil of Qurna and Medynah [In Arabic]. Journal of Arab Gulf, 47, 1-2.
Almutairi, M. S. (2016). Development and Evaluation of a Remediation Strategy for the Oil Lakes of Kuwait (Doctoral Dissertation).
Ashadi, A., Arabbani, F. K., Indriyanti, N. Y., Saputro, S., & Mahardiani, L. (2020). Utilization of Mendong Plant Activated Charcoal as SO2 Gas Adsorbent: Preliminary Study. IOP Conference Series: Materials Science and Engineering, 982, 012023. https://doi.org/10.1088/1757-899x/982/1/012040
Deeri, A. N., & Al-Kaabi, M. H. (2014). Thermal Difference Between Basra City and its Surrounding Towns [In Arabic]. Journal of Geographic Researches, 20 (131).
Ganapathy, V. (1989). Cold End Corrosion: Causes and Cures. Journal of Hydrocarbon Processing.
Ganapathy, V. (2015). Steam Generators and Waste Heat Boilers for Process and Plant Engineers. CRC Press. https://doi.org/10.1201/b17519
Ganguly, R., Vasistha, P., & Gupta, A. K. (2017). Design of an Incinerator to Treat Combined Biomedical Wastes Generated from Four Major Hospitals in Chandigrah and Shimla City, India. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 8(3s), 201.
Hadi, A. H., Fleyh, A. R., Hussain, B. A., Ibrahim, O. M., & Abd Al Razak, A. F. (2023). Power Generation from Utilizing Thermal Energy of Hazardous Waste Incinerators, Journal of Engineering, 29(10), 52-73. https://doi.org/10.31026/j.eng.2023.10.04
IPCC. (2001, June 15). Good Practice and Uncertainty Management in National Greenhouse Gas Inventories [In Arabic]. Chapter 2 Energy.
John, S. E., & Swamy, C. N. (2011). Design of Incinerator for the Treatment of Bio-Medical Solid Waste in Chikmagalur City. Jr. of Industrial Pollution Control, 27(2), 173-179.
Laribi, S., Dubois, L., De Weireld, G., & Thomas, D. (2018). Simultaneous Absorption of SO2 and CO2 from Conventional and Partial Oxy-Fuel Cement Plant Flue Gases. Chemical Engineering Transactions, 69.
Liemans, I., & Thomas, D. (2013). Simultaneous NOx and SOx Reduction from Oxyfuel Exhaust Gases using Acidic Solutions Containing Hydrogen Peroxide. Energy Procedia, 37, 1348-1356. https://doi.org/10.1016/j.egypro.2013.06.010
Shore VAP Water Evaporators (n.d.).
Torero, J. L., Olenick, S. M., Garo, J. P., & Vantelon, J. P. (2003). Determination of the Burning Characteristics of a Slick of Oil on Water. Final MS for SS and TB, 8(4) in Situ Special Issue (Mullin and Champ), 5(2), 1-24. https://doi.org/10.1016/S1353-2561(03)00071-9
Wu, N., Kolb, G., & Torero, J. L. (2000). The Effect of Weathering on the Flammability of a Slick of Crude Oil on a Water Bed. Journal of Combustion Science and Technology, 161, 269-308. https://doi.org/10.1080/00102200008935820
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.