
Biotechnological Interventions for Enhanced Secondary Metabolite Production in Medicinal Plants
Dr. Ragini Sikarwar 1
1 Assistant
Professor and HOD, Department of Botany Government Home Science PG Lead
College, Narmadapuram, (MP), India
|
|
ABSTRACT |
||
|
Medicinal plants are rich repositories of secondary metabolites such as alkaloids, flavonoids, terpenoids, glycosides, and phenolics that serve as essential raw materials for pharmaceuticals, nutraceuticals, and cosmetic industries. However, their natural accumulation in planta is often insufficient, inconsistent, and subject to environmental fluctuations, making large-scale production unsustainable. Biotechnological interventions provide innovative and sustainable solutions to enhance metabolite yield, improve quality, and ensure year-round availability. This review highlights recent advancements in plant tissue culture systems (callus, suspension, and hairy root cultures), elicitor-based strategies, metabolic engineering, synthetic biology, and CRISPR/Cas9-mediated genome editing for enhancing metabolite productivity. The integration of omics platforms (genomics, transcriptomics, proteomics, and metabolomics) has enabled precise identification and regulation of key metabolic nodes. Case studies of Catharanthus roseus, Withania somnifera, and Artemisia annua are discussed to illustrate real-world applications. Although
remarkable progress has been made, challenges remain, including pathway
complexity, high cost of in vitro systems, and regulatory hurdles for
genetically engineered plants. The article concludes that combining
traditional knowledge with modern biotechnology and systems biology will
accelerate the industrial-scale production of bioactive compounds, ensuring
global healthcare sustainability. |
|||
|
Received 07 May 2025 Accepted 08 June
2025 Published 30 July 2025 DOI 10.29121/granthaalayah.v13.i7.2025.6414 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Medicinal Plants, Secondary Metabolites,
Biotechnology, Metabolic Engineering, Hairy Root Culture, CRISPR/Cas9, Omics |
|||
1. INTRODUCTION
Medicinal plants have been the cornerstone of traditional and modern medicine for centuries. Nearly 80% of the global population relies on plant-derived natural products for primary healthcare needs World Health Organization (2019). Secondary metabolites—though not directly involved in plant growth—serve as defense molecules and bioactive agents, offering immense therapeutic potential. For instance, vincristine from Catharanthus roseus is an established anticancer drug, artemisinin from Artemisia annua is the most effective antimalarial, and withanolides from Withania somnifera are potent adaptogens.
Conventional cultivation of medicinal plants suffers from multiple limitations:
· Long life cycles and slow growth rates.
· Seasonal and geographical dependence.
· Low metabolite yield, often below commercial viability.
· Overexploitation of wild species leading to biodiversity loss.
These challenges have stimulated interest in biotechnological methods to produce metabolites in a controlled and reproducible manner. Since the pioneering work of plant tissue culture in the 1950s Murashige and Skoog (1962), to recent CRISPR/Cas9 breakthroughs, the field has undergone exponential growth. This article provides an expanded review of the strategies, applications, advantages, and limitations of biotechnological interventions aimed at enhancing secondary metabolite production in medicinal plants.
2. Methodological Approaches
1) Plant
Tissue Culture Systems:
Plant tissue culture offers a controlled environment for the production of metabolites independent of environmental fluctuations.
· Callus and Cell Suspension Cultures: Used extensively for metabolite production. Suspension cultures are more scalable but face challenges in maintaining metabolite stability Verpoorte et al. (2002).
· Hairy Root Cultures: Induced by Agrobacterium rhizogenes, hairy roots are genetically stable and exhibit faster growth, producing metabolites comparable to parent plants Giri and Narasu (2000).
· Organ Culture: Shoot and root cultures provide targeted metabolite accumulation.
· Case Example: Hairy root cultures of Panax ginseng significantly increased ginsenoside production, which is otherwise low in field-grown plants.
2) Elicitor-Based
Strategies:
Elicitors mimic stress conditions and trigger the plant’s defense system, stimulating biosynthetic pathways.
· Biotic Elicitors: Chitosan, yeast extract, fungal polysaccharides.
· Abiotic Elicitors: UV light, salinity, heavy metals, jasmonic acid, salicylic acid.
· Nanoparticles as Elicitors: Silver nanoparticles have been shown to boost phenolic and flavonoid content in Ocimum sanctum.
· Case Example: Jasmonic acid elicitation in Catharanthus roseus hairy root cultures enhanced alkaloid production up to 20-fold compared to controls Verpoorte et al. (2002).
3) Metabolic
Engineering:
Metabolic engineering involves targeted manipulation of biosynthetic pathways to maximize metabolite flux.
· Overexpression of rate-limiting enzymes (e.g., phenylalanine ammonia-lyase in flavonoid biosynthesis).
· Downregulation of competing pathways using RNAi or gene silencing.
· Engineering precursor supply (e.g., enhancing isoprenoid precursors for terpenoid production).
· Case Example: Overexpression of 3-hydroxy-3-methylglutaryl CoA reductase (HMGR) in Artemisia annua increased artemisinin content by up to 3-fold Zhang et al. (2011).
4) CRISPR/Cas9
and Genome Editing:
Genome editing has revolutionized plant biotechnology by enabling precise modifications.
· Knockout of negative regulators.
· Targeted activation of transcription factors.
· Multiplex genome editing for entire metabolic pathways.
· Case Example: CRISPR/Cas9 editing of SmCYP450 genes in Salvia miltiorrhiza successfully enhanced tanshinone accumulation Li et al. (2017).
5) Synthetic
Biology Approaches:
Synthetic biology reconstructs biosynthetic pathways in heterologous hosts such as yeast and E. coli.
· Entire artemisinin pathway engineered into Saccharomyces cerevisiae, enabling commercial production.
· Modular cloning techniques allow multi-gene assemblies for plant metabolites.
6) Omics-Guided
Pathway Analysis:
Omics technologies provide holistic insights into metabolic regulation.
· Genomics identifies biosynthetic gene clusters.
· Transcriptomics reveals stress-responsive genes.
· Proteomics highlights post-translational modifications.
· Metabolomics maps metabolite flux and bottlenecks.
· Integration of omics with machine learning accelerates pathway discovery and predictive modeling for metabolite enhancement.
3. Results and Discussion
Case Studies of Successful Applications:
· Catharanthus roseus: Jasmonic acid + hairy root cultures boosted vinblastine content.
· Withania somnifera: CRISPR-mediated pathway editing enhanced withanolides.
· Artemisia annua: Transgenic expression of ADS and HMGR increased artemisinin yield.
· Salvia miltiorrhiza: CRISPR editing targeted at CYP450 improved tanshinone levels.
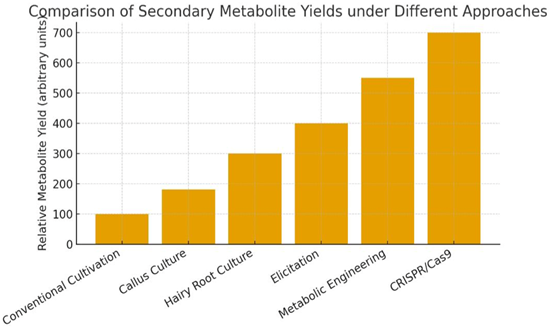
Figure 1
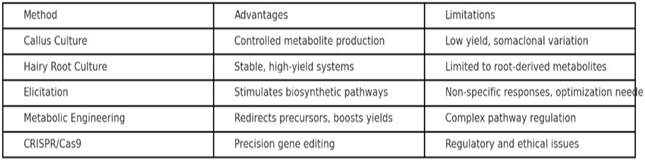
Figure 2
4. Advantages of Biotechnological Interventions
· Sustainability: Year-round production independent of geography.
· Scalability: Bioreactor-based systems.
· Biodiversity Conservation: Reduces pressure on wild populations.
5. Limitations and Challenges
· High setup and operational costs.
· Difficulty in pathway reconstruction due to complexity.
· Consumer and regulatory concerns regarding genetically engineered plants.
6. Conclusion
Biotechnological interventions provide sustainable, scalable, and innovative solutions for enhancing secondary metabolite production in medicinal plants. While plant tissue culture, elicitation, and metabolic engineering have already proven successful, the integration of CRISPR-based editing, omics platforms, and synthetic biology holds unprecedented potential. Future directions should focus on reducing cost barriers, applying AI-driven metabolic modeling, and ensuring global regulatory harmonization. The convergence of traditional knowledge and modern biotechnology will shape the next era of plant-based pharmaceuticals.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Giri, A., & Narasu, M. L. (2000). Transgenic Hairy Roots: Recent Trends and Applications. Biotechnology Advances, 18(1), 1–22. https://doi.org/10.1016/S0734-9750(99)00016-6
Li, M., Zhang, C., Xu, J., Shi, Y., & Yang, X. (2017). CRISPR/Cas9-Mediated Gene Editing in Medicinal Plants. Plant Cell Reports, 36(10), 1447–1456. https://doi.org/10.1007/s00299-017-2202-4
Murashige, T., & Skoog, F. (1962). A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiologia Plantarum, 15(3), 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Van der Heijden, R., Jacobs, D. I., Snoeijer, W., Hallard, D., & Verpoorte, R. (2004). The Catharanthus Alkaloids: Pharmacognosy and Biotechnology. Current Medicinal Chemistry, 11(5), 607–628. https://doi.org/10.2174/0929867043455846
Verpoorte, R., Contin, A., & Memelink, J. (2002). Biotechnology for the Production of Plant Secondary Metabolites. Phytochemistry Reviews, 1, 13–25. https://doi.org/10.1023/A:1015871916833
Zhang, Y., Nowak, G., & Reed, D. W. (2011). Engineering Artemisinin Biosynthesis in Artemisia Annua. Plant Biotechnology Journal, 9(5), 484–494. https://doi.org/10.1111/j.1467-7652.2011.00583.x
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.