
Approaches to Control Biofouling in Potable Water Distribution Networks
Dr. Navpreet Kaur 1
1 Department
of Food Science & Technology Guru Nanak College, Sri Muktsar Sahib, Punjab,
India
|
|
ABSTRACT |
||
|
Water is a critical resource that sustains life, and its availability should be secured.The freshwater is contaminated due to human activities and consequently is enriched in foreign and potentially dangerous species. These pollutants can be classified into biological components that include microbes (bacteria, viruses and fungi), inorganic compounds (radioactive materials and heavy metals), and organic compounds (drugs, soaps, pesticides, fertilizers, and oils) are not only harmful to human health and the environment but also induce changes in natural aqueous habitats and organisms thus affecting the water quality and ecological balance. Fulfilling the
demand for clean drinking water to the general public has been a challenging
task in developing countries. Among various water treatment technologies, the
utilization of nanomaterials and nanostructures has received significant
consideration due to their sustainability and stability. The dimensions of
nanomaterials impart exceptional chemical and physical properties, such as
multivalent interactions with bio-molecular and cellular systems. This paves
the way to treat biofouling of water with nanomaterial due to their
antimicrobial properties and reduces the possibility of harmful disinfection
by-products (DBPs) formation. Different types of nanomaterials that can act
as nanosorbents, nanocatalysts, bioactive nanoparticles, nanostructured catalytic
membranes, nanomembranes and nanoparticles (nanocelluloses) provide an
efficient methodology for solving water bio fouling problems. These highly
efficient nanomaterials owing to the high aspect ratio, surface charge,
surface area and mechanical strength can serve as remediation for biofouling
of water. However, the major issue with nanomaterials synthesized
conventionally is their toxicity although the synthesis of nanomaterials
using green routes can serve as an answer to this problem. |
|||
|
Received 07 December 2024 Accepted 08 January 2025 Published 31 March 2025 DOI 10.29121/granthaalayah.v13.i3.2025.6383 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Biofouling, Water |
|||
1. INTRODUCTION
Water is a vital resource to sustain life and thus is the most basic and irreplaceable need of our ecosystem. Its adequate, safe and accessible supply must be available to all. Improved access to safe drinking water might aid in improved health worldwide. So, every effort should account for safe drinking water that is free of any toxins (chemical, physical or biological) Prest et al.(2016) As per WHO guidelines, safe drinking water does not represent any significant risk to health over lifetime consumption and it includes the different sensitivities that may occur between life stages. Safe drinking water is required for all domestic purposes that include drinking, food preparation and personal hygiene.
The advanced anthropogenic activities have led to an exponential rise in water pollution thus mortifying the quality of drinking water. This polluted water contains heavy metals, pesticides, fertilizers and a high organic load. This puffed-up organic load adds to higher microbial growth within water bodies which accounts for waterborne diseases all over the world and the greatest threat to public health is the consumption of unsafe water-carrying microbes from human and animal excreta. The number of waterborne disease outbreaks have been associated with poor treatment of water supplies and substandard management of drinking water distribution systems Schurer et al. (2019).
As per WHO, in 2020 there were 5.8 billion people (74% of the global population) safely managed drinking-water services while the remaining two billion were without safely managed services. Those who are generally atrisk of waterborne diseases areinfants and children, the elderly or one with sensitive immune responses. The majority of this population belongs to the continents of Asia and Africa.
The differential access to safely managed water services between developed and developing world is due to sharp geographic, socio-cultural and economic inequalities in rural and sub-urban areas. Access to low-quality water-borne services accounts for 8,29,000deaths per year, out of which half are children. The sources of drinking water such as water bodies or groundwater may contain several microbial pathogens which may pose a serious threat to human health. The drinking water might be contaminated with faeces from animals, birds and humans. Faecally derived pathogens are the foremost concern in setting health-based goals for microbial safety WHO (2022). The water might be contaminated during the withdrawal, collection, storage and transportation of water. Importantly, water contamination at the source is minimal as compared to storage spaces. The contamination of these biological infectious entities shoots up with their multiplication and the presence of biofilms in water storage and distribution systems Vavourakis et al. (2020).
Among microbial pathogens, bacterial infection stands out.Bacterial pathogens such as Burkholderiapseudomallei, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Vibrio cholerae, Yersinia enterocolitica, Plesiomonas, Campylobacter spp., Shigella spp., etc.may prove lethal to human systems. These bacterial pathogens may lead to infections such as typhoid, cholera, intestinal infections and many more Abdulrahman (2022).
Since the early twentieth century, conventional methods have been used for water treatment such as coagulation, decontamination, disinfection, desalination, filtration and sedimentation. These processes require large systems, infrastructure and engineering expertise which makes them highly labour-intensive and burdensome. Disinfection is undeniably important in a safe drinking water supply and should be used for both surface and underground water subject to faecal contamination. The destruction of pathogenic microbes ensures their safety to consumers. Furthermore, the chemicals such as chlorine, ammonia, hydrochloric acid, alum, ozone and coagulants can contaminate the fresh water to great extent. Disinfection efficacy may also be unsatisfactory against microbes forming flocs, films or particles which increase their resistance to the disinfectants. Hence, the situation demands low-cost, effective and robust techniques. Here nanotechnology can offer a promising solution as the nanoparticles are known to kill the resistant bacterial species and break down the robust bacterial films which cannot be breached by chemicals Makabenta et al. (2021), Sahli et al. (2022).
2. Clinically Important Bacteria in Water
Waterborne diseases are caused by pathogenic bacteria, parasites and viruses and are responsible for widespread health risks associated with drinking water. The public health burden of disease is calculated by the severity and incidence of the illness caused by the pathogen, its infectivity and microbial load Rani (2021).
Waterborne pathogens have the following properties that differentiate them from other contaminants:
1) These can cause both acute and chronic health effects.
2) These microscopic entities are aggregated or adhered to the suspended solids in the water.
3) The microbes can grow in the water and are discrete.
4) The invasiveness and virulence of the pathogen determine its infectivity.
5) The disease progression in the host depends upon exposure to microbial load and the immune status of the host. Once the disease is established, the microbes multiply in the host.
The presence of different types of these pathogens determines the water microbiology. The microbial growth of these microbes is supported by water. The principal bacterial agents that cause various waterborne infectionssuch as Salmonella typhicausing typhoid fever; Salmonella paratyphi-A responsible for paratyphoid fever; Salmonella (and other serotypes) causative agents of salmonellosis and enteric fever; Vibrio cholerae causing cholera and Pseudomonas aeruginosa leads to various infections. These bacterial agents causing a different type of infectionsthat are summed up in Table 1 Although the mere presence of these bacterial agents is not sufficient to cause the infection. The minimum viable cells (for example a dose of 106-108 Salmonellae per person in strain) are required to elicit the infection though virulence may vary from strain to strain. The infecting dose also varies with the age and immune resistance of the infected population. The most susceptible are infants, the elderly and immuno-compromised patients (Mackenzie et al. (1968), Kumar et al. (2022).
Virulence is a genetic trait Meynell (1961), Brown et al. (2006). The phenotypic expression of virulence may vary even in a clone of the strain. In a given population of cells, a small number of microbes can be unusually virulent Meynell (1961), Brown et al. (2006). Thus, some enteric microbes can infect even if a few numbers of cells are present. Some pathogens are shown to transfer virulence from infectious strains to non-infectious ones thus transferring them to virulent ones. Thus, these virulent factors can be transferred to residential non-infectious intestinal microbial flora.
Table 1
|
Table 1 Water-Borne Microbial Infections |
||
|
Pathogen |
Infection |
References |
|
Vibrio cholerae |
Cholera |
Kumar et al. (2014) |
|
V. alginolyticus |
Soft Skin Infections |
|
|
V.fluvialis V. hollisae V. mimicus |
Diarrhea &
Gastrointestinal Infections |
Abraham (2011) |
|
V. parahaemolyticus |
Gastro-enteritis |
Ottaviani et al. (2012) |
|
V. vulnificus |
Speticemia& Wound
Infection |
Gulig et al. (2005) |
|
Salmonella enterica |
Salmonellosis |
Kumar et al. (2014) |
|
Shigella dysenteria |
Shigellosis |
|
|
S. sonnei |
||
|
S. fiexneri, |
||
|
Escherichia coli |
Gastroentritis,
diarrhea, traveller’s diarrhea |
Kumar et al. (2014) |
|
Enterohemorrhagic E. coli (EHEC strains) e.g., E. coli O157: A |
Bloody diarrhea,
hemolytic uremic syndrome |
Goldwater et al. (2012) |
|
Mycobacterium Avium Complex (Mac) |
Highly resistant
cholera |
Uchiya et al. (2015) |
|
Helicobacter pyroli |
Gastritis (Peptic
& duodenal ulcer disease and gastric carcinoma) |
Graham. (2014) |
|
Aeromonas hydrophyla |
Gastroenteritis,
Septicemia, meningitis and wound infection |
Rao et al. (2022) |
|
Clostridium
perfringens |
Gastroenteritis |
Yonogi et al. (2014) |
|
Leptospira sp. |
Leptospirosis |
Kumar et al. (2014) |
|
Francisellatularensis |
Tularemia |
Maurin (2020) |
|
Yersinia enterocolitica |
Gastroenteritis |
Kumar et al. (2014) |
3. Water Disinfection Processes
The water borne pathogens pose serious global challenges with millions of people losing their life every year worldwide. Biological contamination of water can be curbed with water disinfection techniques. Among various methods, chlorination is preferred method in various developing countries and developed countries because of its simple application and cost effectiveness.Biological pollutants are detoxicated by various physical processes such as adsorption, heating, distillation and filtration; chemical processes like flocculation, light irradiation and photo catalysis and biological processes such as activated sludge. These methods not only battle the microbial pollution but also oxidize iron and manganese in water bodies, enhancing coagulation and filtration activity and eliminating taste and color. The incompetence of these processes has directednecessity of more efficient systems for microbial inactivation as the byproducts of these processes are chlorites, chloral hydrates, dibutyl phthalates, haloacetic acids and haloacetonitriles that are highly mutagenic and carcinogenic. There are around 600 biproducts among whichchlorate tops the chart followed by bromochloro-, bromodichloro-, dibromochloro-, and tribromoacetic acid, trichloronitromethane, and chloral hydrate Muellner et al. (2007). As per Muellner, the limitations of current disinfectants can be enlisted as follows:
1) Short range of anti-microbial activity.
2) Harmful by-products during and after the processes of disinfection.
3) Highly corrosive for surfaces and equipment’s.
4) Rapid degradation needs instant formulations which are tedious methods.
5) Disposal of these products needs biosafety considerations.
6) Storage of such disinfectants is also labor intensive.
7) These techniques are slow, and their repeated use can create resistant microbial flora which needs to be tackled.
The resistant microflora is another major challenge. This free-living microflora can attach to the surface and aggregate to form multicellular communities known as biofilms. These biofilms are made up of extracellular polymeric substancesthat are recalcitrant to antibiotics therapies. These are one of another major cause of recurrent and persistent infections of clinically important microbes such as Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus.
Thus, these conventional techniques of water disinfection with range of issues needs to be replaced by eco-friendly, highly robust and advance technique with high efficiency and efficacy Kooij (2000), Arshad et al. (2019).
4. Nanotechnology
The declining freshwater quality has increased the application of wide range of disinfection processes for producing drinking water. The expensive methods are applied for disinfection of drinking water. Most of modern disinfection processes rely upon physical and chemical treatment technologies. The traditional methods for managing the pathogenic microbes involve the water heating Cabral (2010), Tahir (2019), UV irradiation Tsenter et al. (2022), chlorine and ozone treatment Tony et al.(2016), Zhang et al.(2016), Morrison et al. (2022). These methods are highly effective but may generate excessive secondary pollutants with greater toxicity than parent molecule. Highly toxic elements like trihalomethanes (such as DBP) are formed after chlorine treatment which might cause significant health hazard above 160ppb Bellar et al. (1974), Costet et al. (2011), Singh et al. (2020), Kumari and Gupta (2022). Other conventional systems for water disinfection are filtration through membrane systems. These includes the membrane-based processes like reverse osmosis (RO), nanofiltration and ultrafiltration where the membrane choking is most common problem which decreases the membrane efficiency Mishra et al. (2021), Yu et al. (2022).
The chemical treatments are based on oxidation of organic components in the living cells. However, this particular disinfection is not equally effective especially for the viruses that are less susceptible. The answer to current problem can be solved by nanotechnology. Nanotechnology ismanipulation of materials at the nanometer scale in order to improve and obtain new properties of materials Hornyak et al.(2008), Srinivas (2014), Kumar et al.(2021). Nanomaterials are the materials with dimensions smaller than 100nm (at least one dimension) and can be synthesized by self-assembling of atoms. These nanostructures can be:
1) Zero Dimensional Nanomaterials (0D-NMs): Nanoparticles are zero dimensional structures Grzelczak et al.(2008), Hornyak et al. (2008), Ray et al.(2020), Shen et al.(2023). These have all their dimensions at nano scales such as nanoparticles.
2) One Dimensional Nanomaterial (1D-NMs): Thes nano-materials have two dimesions at nanoscales. Nanorods, belts, fibres, tubes and nanowires are one dimensional structure Sugunan et al. (2006), Khan and Hossain (2022).
3) Two Dimensional Nanomaterials (2D-NMs): These nanomaterials have material thickness at nanoscales. Sheets, plates, thin films, flakes and coatings are two dimensional structures Aoki et al. (2005), Ray et al. (2020), Khan and Hossain (2022). Graphene sheets were first synthesized as 2D-NMs.
4) Three Dimensional Nanomaterials (3D-NMs): 3D-NMs are synthesized using basic units of 0D-NMs, 1D-NMs or 2D-NMs. Arrays, hierarchical structures are three dimensional structures Von et al. (2010), Singh et al. (2020), Khan and Hossain (2022)
This availability of vast range of nanostructures with controlled properties and in nanometer sizes that to similar with most of biomolecules have sparked widespread interest in their application. At nanoscale, these materials possess novel properties that can be used for water disinfection. Some of these applications are based on the high specific surface ratio that adds to fast dissolution, high reactivity and strong sorption while others take advantage of properties such as superparamagnetism, localized surface plasmon resonance, and quantum confinement effect Von et al. (2010), Hajipour et al. (2012), Guo et al. (2013), Philip and Kumar (2022).
5. Synthesis of Nanostructures
Synthesis of nanostructures especially nanoparticles include two main approaches i.e. (1) Top-down synthesis and (2). Bottom-up synthesis. In top-down synthesis, destructive approach is employed. The larger molecule is decomposed to smaller ones which are then converted to suitable nanostructures. Various decomposition techniques used are grinding/milling, CVD (Chemical VapourDeposition), Physical VapourDeposition, Sonication etc. Abid et al. (2022), Gutiérrez et al. (2022). This approach was used to synthesize magnetite nanoparticles from natural iron oxide (Fe2O3). The size of nanoparticles formed in presence of oleic acid was reported to be 20nm to 50nm Pal (2020), de et al. (2022).
Another method is Bottom-up synthesis.Here,nanomaterials are formed from simpler substances thus known as bottom-up approach. Various techniques employed in bottom up approach are sedimentation and reduction techniques including sol gel, green synthesis, spinning and biochemical synthesis Nkele and Ezema (2020), Guan et al.(2022), Samuel et al.(2022). Nanoparticles based TiO2 anatase were synthesized. These TiO2 based nanoparticles had graphene domainsand were synthesized using alizarin and titanium isopropoxide precursors. Well uniform spherical nanoparticles were synthesized. More recently, green and biogenic bottom-up synthesis is being followed by many researchers as this process is more feasible and less toxic in nature. The synthesis of nanomaterials is achieved via biological systems such as plant extracts, bacteria, yeast, fungi and human cells making them more cost effective and nature friendly Shah et al. (2021), Noah and Ndangili (2022).
5.1. Applications
The recent advances in nano sciences offer surmount opportunities for treatment of water and water systems. Nanotechnology enabled water treatment systems to overcome the major challenges faced by existing treatment technologies Qu et al. (2013).
The antibacterial properties of various nanostructure vary from one nanoparticle to another.
1) Carbon
Based Nano-Adsorbents
Carbon is one of most abundant and versatile elements on the planet. It has wide range of applications.Carbon nanomaterials are known for their sorption qualities due to their unique structures and electronic properties. These can adsorb wide range of contaminants with faster kinetic rates and also have large surface area. There are array of carbon nanomaterials ranging from carbon nanotubes (CNTs) to carbon beads. Among these fullerenes and CNTsare most widely scrutinized due to their stability Arshad et al. (2019).
2) Fullerenes
Fullerenes are the allotropes of carbon where the carbon atoms are sp2hybridized to form globular hollow cage like nanostructurewith various shapes (hollow sphere, ellipsoid etc.)as shown in Figure 1. The spherical fullerene is known as buckminsterfullerene (C60; bucky balls or bucky onions). C60 is primary structure of all fullerenes. The cage like fused ring shape is made up by twenty hexagons and twelve pentagons. The ‘buckyballs’ is the favorably regarded as it has ability to form the derivatives of fullerenes and nanocomposites. The derivatives of fullerenes range from diphosphonate, phosphonates to organophosphorus compounds. However, the fullerenes lag at solubility and nearly insoluble in organic solvents such as toluene and carbon disulphide. Theirmoderate solubility at room temperature has been put to use. These can be synthesized via various methods such as arc discharge, CVD, laser ablation, laser irradiation of polyaromatic hydrocarbons (PAHs) and resistive arc heating of graphite.Fullerenes and their derivatives have outstandingfunctions as antioxidants, biopharmaceutical compounds, catalysts, organic photovoltaics and water purification/ bio-hazard protection catalysts Shah et al.(2021).
These nanostructures testified to have high electron affinity, high electrical conductivity, high strength, stable structure and are highly versatile Astefanei et al. (2015), Kurosawa et al. (2021). Pentacatonic fullerenes designed by Thota and co-workers had high water solubility and were found to be effective against both gram-positive and gram-negative bacteria Thota et al. (2012).
Fullerenes can also be substituted with polyhydroxyl groups to generate fullerenols. These fullerenols have shown high antioxidant activity due to increase in number of oxygen atoms. These nanoparticles have high specific area and chemically incorporated antibacterial action making them attraction to researchers in last few years Shah et al. (2021).
Figure 1
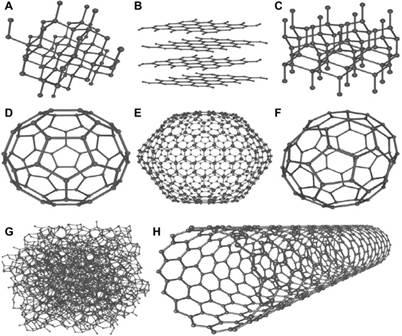
|
Figure 1 The Different Allotropes of carbon: (A) Diamond (B) Graphite, (C) Lonsdaleite, (D) C60 (Buckminsterfullerene or Bukyball), (E) C540 Fullerene, (F) C70 Fullerene, (G) Amorphous carbon, (H) Single-walled carbon nanotube Aqel et al. (2012) |
3) Fullerene
Composites
The fullerene/polystyrene film composites were found to be effective against various bacteria like Staphylococcus aureus, E. coli and Candida albicans. The polystyrene film alone was however unable to exhibit such bacteriostatic properties while clear zone lacking microbial growth was observed in composites Alekseeva et al. (n.d.)These characteristics are imparted to composites by the fullerenes. In another research, fullerene composites with cellulose, chitosan and γ cyclodextrin were reported to have antimicrobial activity against vancomycin-resistant enterococci Duri et al.(2017) A photostable and photodynamic nanocomposite of fullerene (C60) was created in which fullerene C60 is covalently linked to ethylenedioxythiophene (EDOT) and this was found to be effective against Staphylococcus aureus with 99.9 % reduction in bacterial populations after visible light irradiations Reynoso et al. (2021). Ballatore and coworkers evaluated the microbial inactivation by porphyrin-fullerene C60 polymeric rings. These nanocomposites displayed excellent phototoxic features against S. aureus and E. coli. The scientist also substituted the porphyrin-fullerene C60 by carbazoyl groups. These modified nanocomposites were capable of inactivating S. aureus after 30 minutes of irradiation Ballatore et al. (2022). Thus, fullerene nanocomposites feature a fascinating, flexible, photodynamic and active surface that can produce ROS and hence can annihilate microbes.
4) Carbon
Nanotubes (CNTs)
CNTs are graphene cylinders with diameter of unit nanometer Ibrahim et al. (2019). CNTs have been predicted as metallic or semiconducting in nature Aqel et al. (2012), Peng et al.(2019), Obeid and Sun(2022) .CNTs are graphite sheets rolled in itself Figure 1 and Figure 2. These rolled sheets can be single walled or double walled, hence classified as below on basis of their structure:
1) The single layer graphene sheets rolled up into cylinders (SWCNTs)
2) The two-layergraphene concentric cylinders (DWCNTs)
3) The multiple layered concentric cylinders known as multiwalled carbon nanotubes (MWCNTs).
Figure 2
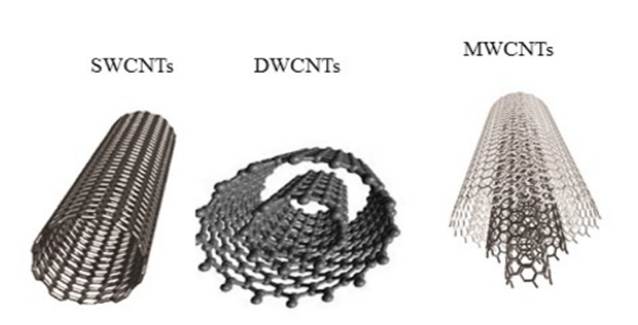
|
Figure 2 Variable Structures of Carbon Nanomaterials (CNTs). SWCNTs: Single-Wall Carbon Nanotubes; DWCNTs: Double-Walled Carbon Nanotubes; MWCNTs: Multi-Wall Carbon Nanotubes. Azizi-Lalabadi et al. (2020). |
CNTs are known to accelerate the transfer of electrons between electro-active species and electrodes Zhou et al. (2019) and proven to have elevated adsorptive ability Mubarak et al. (2016), conductivity Saifuddin et al.(2013) and high surface to volume ratio Yadav et al. (2020). The applications of CNTs in nanotechnology range from drug delivery, gene manipulation, tissue engineering to antimicrobial agents. The major fall back is insolubility of CNTs in various solvents thus limiting its usage which can although be altered with various modifications. The preferred methods for synthesis of CNTs are arc discharge process, laser ablation, chemical vapor deposition (CVD) and ball milling, Azizi-Lalabadi et al. (2020). Due to unique physical and chemical properties such as super adsorption, CNTs has been used as an antibacterial agent. The size of these nanomaterials plays an outstanding role in microbial deactivation. The further decrease in size increases their surface to volume ratio consequently their strong bond with cell envelopes leading to disruption of cellular membrane, metabolic processes and morphological structure Khan et al. (2016). These antibacterial properties are observed due to direct contact of CNTs to cell membrane which results in cell death via changes in membrane fluidity, oxidative stress, enzyme inhibition, and reduced transcription of several key genes. The antimicrobial activity of CNTs is strongly related to their aggregation degree, concentration, degree of purification diameter, length, surface functionalization and time and intensity of contact. The addition of metals and other supports to CNTs improves the adsorption, mechanical, optical and electrical properties of nanotubes. Apart from cellular destruction with decrease in size of CNTs the increase in cellular efflux was observed Maksimova (2019). The other properties like surface area and dispersibility can be enhanced by increasing the number of oxygen, nitrogen and other specific groups attached to the surface Karim et al. (2017), Perazzoli et al.(2017), Mbiri et al. (2018).
The first CNTs were single walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes were latter synthesized by Kang and co-workers in 2007. These CNTs effectively removed various microbial cells such as Escherichia coli, Micrococcus lysodeikticus, Streptococcus mutans, E. coli, Salmonella spp. cells Kang et al. (2007), Liu et al. (2018), Maksimova (2019). The cytotoxicity of CNTs is affected by the various physiochemical properties of these nanomaterials and their transport behaviour in the solution. The antibacterial efficiency may vary with the size and diameter of CNTs, their dispersing ability in the culture medium, dosage, reaction time and mode of action between the bacteria and CNTs. The workers further observed that the size of these particles affect their activity in 2008. Both SWCNTs and MWCNTs were tried against microorganisms and SWCNTs were found to be much more effective by reason of active surface area. The toxicity of CNTs is based on their contact with cell surface. Therefore, diameter of CNTs is an imperative factor in the microbial cell deactivation. The SWCNTs were found to be more toxic owing to higher surface area, better penetration into the cell because of smaller diameter, unique chemical and electronic characteristics and their role in changing the expression of stress related genes. Indeed, SWCNTs offers substantial number of antibacterial properties against both Gram-negative and Gram-positive bacteria. However, MWCNTs lag in such bacteriostatic properties. SWCNTs connect to cell wall thereby aggregating and thus inducing cell wall damage. The surface charge on CNTs is also related to antibacterial activity as the bacterial growth is deterred by generation of reactive oxygen species Bing et al. (2016). The aggregation and agglomeration between the cells is supported by van der Waals forces. The higher concentration of SWCNTs proved to be highly antimicrobial versus Salmonella enterica and E. coli .The length of CNTs also impact the antimicrobial properties Yang et al. (2020). The increase in length of SWCNTs increases the aggregation and antimicrobial properties. CNTs can prevent formation of biofilms by preventing the adherence of cells to surface. CNTs are found to be more beneficial against biofilms if introduced at earlier stages of biofilm production.
Another excellent property of CNTs is adsorption. Carbon nanotubes are highly efficient adsorbents for organic chemicals than active carbon Pan and Xing (2008), Zhang et al. (2019). This is mainly due to high surface area (external surface area) of CNTs and their interactions with the biological contaminants Yang and Xing (2010). The CNTs have high adsorption capacity for bulky organic molecules because of their larger pores in the bundles and have more accessible adsorption sites than activated carbon which on the other hand contains micropores are inaccessible to bulky organic molecules such as many antibiotics and pharmaceuticals compounds Pan and Xing (2008). CNTs are also known to adsorb low molecular weight polar organic compounds. These nanoparticles penetrate the cell envelopes and thus alter the cellular mechanisms both at the molecular and biochemical levels. These CNT-contaminant interactions can be hydrophobic, pep interaction, hydrogen bonding, covalent bonding or electrostatic interactions Yang and Xing (2010), Dubey et al. (2021). These are excellent adsorbents with high adsorption specificity for various contaminants such as dichlorobenzene Wang et al.(2016), Zn2+ Tang and Wang (2018), Pb2+, Cu2+, and Cd2+ He et al.(2019), ethyl benzene Yin et al.(2020), and dyes Quirós et al. (2015).
The major limitation in its application is the development of CNTs which is a costly affair. To decrease the cost of synthesis composite nanotubes were synthesized. These were prepared by combining the adsorption properties of CNTs with magnetic properties of iron oxide, thus aiding their easy recovery and reusability.Apart from synthesis, their limited water dispersal decreases the interaction of CNTs with microbes hence might lower efficacy of CNTs Yang and Xing (2010), Dubey et al. (2021).
The derivatization and attachment of functional groups to the nanoparticles and CNTs might change their biological effects including toxicity. The nanoparticle related long-term injuries can be associated with their accumulation in organs like lungs, liver and spleen. These are also known to cross the blood-air barriers, blood-alveolus barrier, blood-brain barriers and blood-placenta barrier, hence causing major injuries. The toxicological effects include apoptosis, autophagy, inflammatory response and necrosis Tang and Wang (2018), Wu et al. (2021)
5) CNT
Composites
The carbon nanomaterials composites with biopolymers and NPs like Ag, CuO, TiO2, ZnO have assured antimicrobial effects Ahmad et al. (2019), Azizi-Lalabadi et al. (2020) Carbon nanomaterials can be functionalized with various chemical groups that adds to dispersion capability of these materials. The powerful synergistic effect has been observed in CNTs composite. Dong and co-workers proved that use of Ag and CNT composite enhances bactericidal properties than CNT alone. This effect is enhanced due to their mechanisms that disrupt the cell membranes which ease the penetration of other nano-molecules into the cells. In another research, AgNPs (Silver Nanoparticles) were deposited on MWCNTs along with polyamidoamine. These nanocomposites induced bacteriostatic effects against S. aureus, E. coli and Pseudomonas aeruginosa Yuan et al. (2018). A good example of synergistic effect is study of CNT/poly(L-lysine) or poly (L-glutamic acid) composite film where population of E. coli and Staphylococcus epidermis was reduced by 90% Aslan et al. (2012). The composites of chitosan with CNTs were reported to be efficient against the removal of E.coli and Candida tropicalis. With increase in the concentration of CNTs the bacteriostatic properties of composites are also enhanced. These composites displayed a well-defined porous structure with high water uptake and efficiency Xia et al. (2018). In 2019, Beningo along with co-workers conducted research involving low-polyethylene (LDPE)-based nanocomposites containing MWCNTs that exhibited remarkable antimicrobial activity against DH5α E. coli. The effect of these nanocomposites was also studied on biofilms.
6) Graphene
and Graphene Oxide
Graphite is two dimensional naturally occurring crystalline carbon material or layers of graphene molecules lying upon one another. The carbon atoms are sp2 hybridized in a hexagonal network forming graphite molecules. Each of graphite plate is connected to another by Van der Waals forces. Geim along with coworkers introduced the two-dimensional graphene in 2004 that exhibits exceptionally high crystal and electronic quality. The graphene and graphene oxide were reported to have ample of applications in range of fields. The graphene oxide is an intermediate product of the graphene chemical synthesis. It consists of graphene molecule functionalized with groups such as hydroxyls, carboxyls, carbonyls and epioxides Geim and Novoselov (2007), Jin et al. (2020). In fact, graphene oxide (GO) and reduced graphene oxide (rGO) are the two products which are formed. Among which rGO has been reported to have strong barrier properties against He, H2, water vapor, NaCl and hydrofluoric acid in films. Graphene has ample applications in electronics, medicine and technology. The most successful application of graphene and its molecules has been reported in sensor technology, however antimicrobial applications have also been scrutinized. This antimicrobial nature of graphene and graphene oxide is due to superlative features such as extensive surface areas aswell as unique thermal, electrical and physio mechanical nature Azizi-Lalabadi et al. (2020).The physiochemical properties such as aggregation, arrangement mode, dispersibility, edges, layer numbers, shape, sheet size and surface functionalization affect the antibacterial nature of graphene and graphene oxide nanomaterials Omran and Baek (2022)
The production of graphene and graphene oxide is simple, fast and cheap and it have minimal toxicity on mammalian cells Bolotin et al. (2008). The graphene and graphene oxide interact the cell membrane and cell wall through reactive oxygen species through physical demolition and chemical oxidation Akhavan and Ghaderi (2010), Dolati et al. (2023). The graphene-based nanomaterials have been reported to effectively inhibit the growth of E. coli bacteria while minimum toxicity was reported Hu et al. (2010). In another study, the graphene and graphene oxide sheets could entrap the bacterial cells and thus preventing their replication Akhavan et al. (2011). Khan and coworkers synthesized graphene oxide/carbon nanotube/poly (O-toluidine) (GO-CNT-POT) nanocomposite that was found to effective against Gram positive bacteria Bacillussubtilis and Gram-negative bacteria Escherichia coli. Graphene oxide was reported to have high antimicrobial activity than reduced graphene oxide particles Khan et al. (2016), Godoy-Gallardo et al. (2021). Thus, above mentioned allotropes of carbon can be considered as potent antimicrobial agents and can be used for water disinfection processes owing to high efficacy and low toxicity.
6. Metal-Nanoparticles
Microbial resistance to most of antibiotics and anti-microbial agents decreases the efficacy of eradication methods. So, development of new methods seems to be of supreme importance. Metal nanoparticles and their oxides such as silver nanoparticles (AgNPs), Titanium nanoparticles (Ti/ TiO2 NPs), Iron nanoparticles (Fe/Fe oxide NPs) and Zinc nanoparticles (Zn/ZnO NPs) have been applied as an antimicrobial agent Palza (2015), Brandelli et al. (2017), Hoseinzadeh et al. (2017), Sánchez-López et al. (2020), Ribeiro et al. (2022). Metal precursors forms the basis of these metal nanoparticles. The antimicrobial efficiency and efficacy of these nanoparticles is governed by type of materials used for NPs preparation, facet, particle size and shape Seil and Webster (2012), Roy et al. (2013), Agnihotri et al. (2014), Saied et al. (2022). With decrease in the particle size, the surface/volume ratio is increased that noticeably increases the dissolution rate, catalytic activity, heat treatment and mass transfer thus increasing the bactericidal effects of nanoparticles Shaterabadi et al. (2022). The possible mechanisms were proposed for antimicrobial effects of metal nanoparticles:
(a) the free metal ions dissolute from surface of NPs leading to toxicity
(b), via formation of reactive oxygen species (ROS) that causes oxidative stress Zhang et al. (2013a), Sarfraz et al. (2020), Kessler et al. (2022)
These NPs also possess unique optical properties that aids to surface plasmon resonance. NPs of metals like Cu, Ag, Pt and Au have broad absorption bands in the visible range of the electromagnetic spectrum. These advanced optical properties of metal nanoparticles make them preferred choice for much research-based applications Khan et al. (2019). The metal nanoparticles can be functionalized with antibodies, peptides, RNA and DNA to target different biological systems such as microorganisms, protozoa and viruses. These metal nanoparticles can be classified on the basis of valency such as:
6.1. Zero-Valent Metal Nanoparticles
Metal based nanoparticles such as zinc, silver, iron etc. are effective methods for water disinfection. These metal oxides are also known to adsorb heavy metals and radionuclides Koeppenkastrop and De Carlo (1993), Kumar et al. (2020)
6.1.1. Silver Nanoparticles
Silver nanoparticles are most common inorganic nanoparticles used as an antimicrobial agent against wide range of bacteria. The highly toxic Silver Nanoparticles (AgNPs) have been widely used for disinfection of water due to its good antimicrobial properties against broad range of microbes, including viruses’ bacteria, and fungi Homem and Santos (2011), Wols and Hofman-Caris (2012), Luo et al. (2014), Bag et al. (2021), Yu et al. (2022). The mechanism of antimicrobial action is still debatable; however, range of theories were put forth. AgNPs are reported to act in following ways:
1) AgNPs triggers the release of free radicals and oxygen reactive species when AgNPscome in contact with the bacterial surface. These free radicals damage the cell membrane leading to cell death Siddiqi and Rao (2018).
2) AgNPs are reported to adhere to the bacterial cell wall and subsequently penetrate it. This adherence results in structural changes within the membrane of cell hence magnifying its permeability to various other antimicrobial substances Ng et al. (2019).
3) AgNPs are reported to release Ag+ ions on dissolution which interacts with the thiol groups of many vital enzymes. These interactions inactivate the enzymes by disrupting the thiol bonding, subsequently altering normal metabolic balance of the cell Wanda et al. (2017).
4) Another explanation of cell death by AgNPs is by their interaction with sulphur and phosphorus elements in DNA. This interplay results DNA destruction Sousa et al. (2018).
In recent years, AgNPs have been successfully applied to the treatment of drinking water. The silver nanoparticles successfully eliminated S. aureus and E. coli. The efficiency of removal was enhanced with decrease in size of nanostructures and were less toxic than synthetic fungicides. The shape of AgNPs also play vital role in determining the antibacterial efficiency. Among various shapes such as spherical, rod shaped and truncated triangular, latter was found to be most effective against S. epidermis, Pseudomonas aeruginosa and B. megaterium due to high atom density surface Pal (2020). However, the direct application of these nanomaterials is limited because of AgNPs tend to aggregate in aqueous media Benstoem et al. (2017). These aggregations reduce the long-term efficiency of nanomaterials. This process has been proved to be more efficient and cost-effective when nanoparticles are attached to the filter material Gerbersdorf et al. (2015). This can be achieved bydepositing silver from silver nitrate by its reduction on to cellulose membranes. These AgNPs sheets were capable of inactivating Escherichia coli and Enterococcus faecalis in water samples with low silver loss from sheets. Therefore, AgNPs sheets can prove to be effectiveemergency water treatment solution Gavrilescu et al. (2015).
In another study, chitosan cryogels decorated with silver nanoparticles were reported to have efficient bactericidal capacity against Escherichia coli and Bacillus subtlis Fan et al. (2018) . Silver nanoparticles immobilized in glass capillary tubes were used for water disinfection in fix bed reactor. The AgNPs can also be incorporated to polyethersulfone (PES) microfiltration membranes via chemical reduction. These membranes exhibited strong antimicrobial properties nearby the membranes, thus can have great potential for water treatment application. The microfiltration membranes modified with silver nanoparticles inhibits the growth of microbial films thus potentially increasing the biofouling resistance Yu et al. (2021).
AgNPs also have been successfully applied to ceramic filters made up of clay and sawdust. It was also reported that colloidal AgNPs enhanced the filter performance for E. coli removal with rates as high as 97.8% and 100% Hennebel et al. (2012). The ceramic membranes exhibited remarkable household water disinfection properties. The membranes were capable of efficiently removing Escherichia coli bacteria Barbosa et al. (2016), Ali et al. (2019).
Although the use of AgNPs appears to be attractive due to antibacterial properties, however the presence of these in potable water may have substantial health effects asthese small size nanoparticles are highly reactive species.
6.1.2. Iron Nanoparticles
Iron nanoparticles are extensively scrutinized among zero-valent metal nanoparticles as these possess excellent adsorption properties, are of low cost and could be easily recovered under the influence of magnetic field. The reduction potential of iron is low because of low standard reduction potential. Fe0 under aerobic conditions is oxidized by H2O or H+ to generate Fe2+ and H2, both of which are potentially reducing agents for contaminants. Fe2+ is further oxidized to Fe3+, that forms Fe (OH)3 with increase inthepH.Fe(OH)3 is a flocculent thus removes the contaminants. These redox reactions also generate the free radicals that have strong oxidizing potential for range of organic compounds Hopkins et al. (2016).
Hong and co-workers reported the efficient removal of E.coli with 100% sterilization by use of magnetic iron oxide nanoparticles immobilized with sugar containing poly (ionic liquid). Zaki and co-workers synthesized green iron nanoparticles in 2019 under aerobic and anaerobic conditions via nitrate reductase. These green nanoparticles were effective against algal and biofilm formations in real water (fresh, sea and salt) and wastewater (municipal, agricultural and industrial). Inanother study, PEG coated nanoparticles functionalized with antimicrobial peptide was found to be effective against E. coli K-12 DSM 498 and Bacillus subtilis with minimum inhibitory concentration of 500µm for both of the strains Zaki et al. (2019).
6.1.3. Zinc Nanoparticles
Zn has more negative reduction potential than iron thus is a stronger reductant compared to Fe. Hence it can be used to treat water for removal of contaminants from it.Fe doped ZnO nanoparticles were investigated for disinfection capability against multi drug resistant E.coli from river, pond and municipal tap Duri et al. (2017). In another study, alginate beads encapsulated with ZnO were examined for inactivationof Staphylococcus aureus in both synthetic and surface water and were reported to remove 200 cfu/ml of bacteria within70 minutes of exposure Motshekga et al. (2018).
Munnawar along with co-workers in 2017 scrutinized antifouling polyethersulfonate membranes for water disinfection with fabricated Chitosan Zinc oxide hybrid nanoparticles. The membranes were obtained to have significant antibacterial aswell as antifungal properties due to synergistic effect of chitosan and ZnO against range of bacteria and fungi such as S. aureus, B. cereus, E. coli, S. typhi and A fumigatus.
6.2. Metal Oxides Nanoparticles
6.2.1. TiO2 Nanoparticles
Figure 3
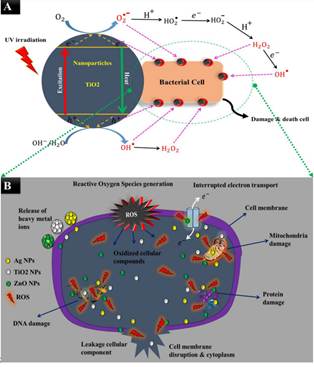
|
Figure 3 Mechanisms of Antimicrobial Activity of Nanoparticles (B). Nanoparticles Generate free Radicals that Causes Oxidative Stress (i.e., ROS) (A). The ROS Destroy the Morphological Structure of Cell by Efflux of Cell Materials Sharmin et al. (2021) |
The crystal structure, size and shape impart the antimicrobial activity to TiO2 nanoparticles. The NPs create oxidative stress by generating reactive oxygen species (ROS) such as hydroxyl radicals and hydrogen peroxide that causes the site-specific DNA damage Zhang et al. (2013b), Maksimchuk et al. (2020). The spores, particularly bacterial endospores, fungal spores and protozoan cysts, in resting stages and are more resistant due to increased cell wall thickness. The cell death by TiO2 nanoparticles involves the degradation of cell wall and cytoplasmic membranes by ROS which initially leads to efflux of cellular contents which may be followed by complete mineralization of the cell. The mechanism involves photoexcitation of TiO2 surface to generate electron-hole pairs that migrate to TiO2 surface Mohammed Sadiq et al. (2010). The photogenerated holes in TiO2 can react with adsorbed water or hydroxylions at the catalyst/water surface to produce the highly reactive hydroxyl ions and electrons that react with oxygen to form superoxide ions. The mechanism of action is depicted in Figure 3.
The wide range of micro-organisms such as Gram-positive and Gram-negative bacteria, fungi, algae, protozoa and viruses can be removed by photocatalytic degradation. The presence of catalyst along with light trigger the oxidation eventually transforming these into CO2 and H2O. TiO2 has high photo-catalytic activity and photostability hence most widely preferred photocatalyst among various semi-conductors. It is also reported to has good chemical and biological stability Chauhan et al. (2019), Ibrahim et al. (2019), Zhang et al. (2019). The TiO2 absorbs in ultraviolet region. This absorption generates reactive oxygen species (ROS) which act upon the microbes. However, this absorption in UV-region is the limiting factor for nanoparticles. Thus, research has been conducted to improve the photocatalysis in the visible range was reported to be enhanced by metal doping which inactivated the bacteriaand viruses Adeleye et al. (2018), Peng et al. (2019). For example, among metal doping, Ag has been widely studied and it improves the visible light absorbance of TiO2 NPs Anirudhan and Deepa (2017), Zhao et al. (2018). Another report of C-TiO2 nanoparticles synthesized by sol gel methods photochemically inactivated Listeria monocytogenes Shim et al. (2016), Piatkowska et al. (2021). Some nonmetals elements such as N, F, S and C have also been reported to narrow the band gap significantly, enhancingthe adsorption in visible region Fahiminia et al. (2019), Wang et al. (2021). Also, H2O2titanium dioxide suspensions have been used against Staphylococcus epidermis biofilms disinfections.
Besides, the production process for TiTO2 based Nanoparticles is rather difficult, hence these should be recovered from wastewater. These particles can be coupled to membranes such as poly(vinylidene fluoride) Park et al. (2018), Huang et al. (2019), Yang et al. (2020), polyethersulfone Fang et al. (2017), Park et al. (2018), polymethyl methacrylate, and poly(amide-imide) Zhang et al. (2018), Pandiyan et al. (2019). This coupling of NPs to membranes aids in its separation by simple filtration processes. Another solution to this problem is by doping magnetic nanoparticles which can be trapped by magnetic traps Coker et al. (2012), Akhil et al. (2016), Stueber et al. (2021).
6.2.2. ZnO Nanoparticles
ZnO NPs have strong oxidation ability and photocatalytic property Fakhriet al. (2018), Trawiński and Skibiński(2019) making them one of the preferred choices for water treatment. These are eco-friendly as they are compatible with organisms Islam et al. (2018). Both TiO2 and ZnO NPs have similar band gap energies but latter ones have low cost over the other. Being similar to TiO2 NPs the light absorption is limited to ultraviolet region. Besides, they have low photocatalytic efficiency as these might be impeded by photocorrosion (Nogueira et al., 2018). This can be greatly enhanced by metal doping with various types of anionic dopants, cationic dopants, rare-earth dopants, and codopants Nogueira et al. (2015), Lee et al.(2016). Also, coupling with other semiconductors, such as CdO, CeO2, SnO2, TiO2, graphene oxide (GO), and reduced graphene oxide (RGO), has also been reported to enhance the photocatalytic efficiency of ZnO NPs Lee et al. (2017) , Xiang et al. (2017), Jiao et al. (2018), Li et al. (2018), Neamtu et al. (2018), Zhao et al. (2018), Zheng et al. (2022).
The Zinc Oxide (ZnO) is safe for human skin which make it proper additive in water disinfection Schilling et al. (2010); Ayyaru et al. (2020), Deka et al. (2022). Li and workers in 2018, reported antibacterial effects of ZnO on gram-positive and gram-negative bacteria as well as on high temperature and pressure resistant spores.The antibacterial efficiency is enhanced by concentration increase and size reduction The vi Rasheed et al.(2020)ability of microbes is believed to be decreased by one of the following mechanisms. One proposed is generation of H2O2 particles while another study reported the accumulation of nanoparticles on the bacterial cell surface due to electrostatic effects Hussein et al. (2019). The other reasons being the ROS generation from the surface of the particles, zinc ion release, membrane dysfunction and nanoparticle internalization. ZnO NPs have bacteriostatic effects against wide range of bacteria such as P. aeruginosa, L plantarum, L. monocytogenes E. coli, S. choleraesuis, S. aureus, Saccharomyces cerevisiae, A. niger, C jejuni Xie et al. (2020), da et al. (2019), Gudkov et al. (2021)
6.2.3. Iron Oxides Nanoparticles
The ease of recovery duetomagnetic properties of iron it is also preferred for water treatment processes. The iron oxides such as magnetic magnetite (Fe3O4) and magnetic maghemite (γ-Fe2O4) and nonmagnetic hematite (α-Fe2O3) can be used as nanoadsorbents for heavy metals. The recovery of small sized nanosorbent NPs can be easily assisted with external magnetic field Chen and Li (2016), Sharma and Feng (2019), Rasheed et al. (2020)
Ferrate based nanoparticleswith physiochemical properties like oxidatioin, co-agulation and disinfection were studied for elimination of diverse range of chemical and biological species from, water/waste water samples Rai et al. (2018) . In another study, iron oxide nanoparticles have been functionalized with range of ligands such as ethylenediamine tetraacetic acid (EDTA), L-glutathione (GSH), mercaptobutyric acid (MBA), α-thio-ω-(propionic acid) hepta(ethylene glycol) (PEG-SH), and meso-2,3-dimercaptosuccinic acid (DMSA) Rojas and Horcajada (2020) or polymers (e.g., copolymers of acrylic acid and crotonic acid) Ma et al. (2019) that are reported to enhance their adsorption efficiency. The ligand polymers form a flexible shell that facilitates the incorporation of various functional groups. This shell prevents the aggregation of particles and improves the dispersion stability of nanostructures Zeng et al. (2015). The polymer molecules not only ensure the intactness of properties of Fe3O4 nanoparticles but also binds with the metal ions thus acting as carriers of metals Ma et al. (2019).
Among various iron oxide nanoparticles, hematite is stable and cheap thus can be used in catalysis and sensors Zeng et al. (2015). Nanohematite particles have been reported to be an effective adsorbent from heavy metal spiked tap water.The enhanced surface area with multiple spaces and pores was reported in flower-like α-Fe2O3 structures. This aided in better removal of As (V) and Cr (VI) from water than previous studies Wang et al. (2018).
7. Ceramic Based Nanomaterials
Ceramic based nanoparticles are synthesized from nonmetallic solids via heat and successive cooling Sigmund et al. (2006), Punia et al. (2021). These are amorphous, polycrystalline, dense, porous or hollow structure have been successfully applied to catalysis, photocatalysis, photodegradation Thomas et al. (2015), Vinayagasundaram et al.(2023). These ceramic based nanoparticles can be used for water disinfection processes.
8. Nanomaterials with Semiconducting Properties
Semiconducting materials possess properties of both metals and non-metals. Semiconductors possess wide bandgaps and therefore were reported to show significant alteration in their properties with bandgap tuning. These can be thus proveto be landmark for futuristic water disinfection processes.
9. Polymeric Nanoparticles
These polymeric nanoparticles are organic based NPs that are mostly nanospheres or nanocapsular in shape Zielińska et al. (2020). The nanospheres have solid matrix particles with other molecules adsorbed at the outer spherical surface while in nanocapsule the solid mass is encapsulated within the particle Rao and Geckeler (2011), Sharma et al. (2020).These PNPs can be functionalized and thus have multiple application. In one such study, macroporus methacrylic acid copolymer beads were prepared with silver nanoparticles adsorbed to its surface. These polymeric molecules were tested to be effective against both gram-positive and gram-negative bacteria Zaharia et al. (2022).
10. Nanocomposites
The various drawbacks of nanomaterials can be overcome by synthesizing the composites of nanoparticles where mixing of two or more nanomaterials are combined for different properties. For example, the chemical deposition of nZVIon CNTs imparted good adsorbent properties and magnetic properties adding to both efficiency of contaminant (nitrate) removal and recovery of nanocomposites Awasthi et al. (2019).
Utilization of spinel ferrite nanocomposites (SFNCs) for water purification either as photocatalyst or as an adsorbent is considered as one of the best cost-effective, ecofriendly and simple technology Kefeni et al. (2017). The application of chitosan nanocomposites has also been extensively studied, for their efficiency in treating wastewater as they are good chelating agents, absorbents and support other nano size particles. Chitosan nanocomposites possess remarkable antimicrobial, biodegradable and non-toxic properties Babaei et al. (2021).
Copper ferrite nanocomposites (CuFe2O4) have also attracted the attention of many researchers due to their potential application in water treatment. CuFe2O4 is an important spinel ferrite, because of its capability to change its physical characteristics, which range from magnetic, electrical, electrical switching and semiconducting properties when they are exhibited under different experimental conditions. CuFe2O4 nanocomposites possess good magnetic properties and are stable under various environmental states. The overall cost of the water treatment process is lowered by the use of CuFe2O4 nanocomposites, as they require visible light as an energy source and can also be reused many times Hosseini et al.(2022).
In last few years, extensive research has been conducted to develop efficient and selective polymeric membranes and adsorbents. Polymeric nanocomposites consist of nanoparticles dispersed in polymeric matrices such as cellulose, resins, dendrimer, etc., to improve their physicochemical, thermophysical, and mechanical properties. Polymer nanocomposites can be developed by using various methods, and the most prevalent techniques are in-situ polymerization, melt-mixing, mixing, selective laser sintering and electrospinning. Polymer nanocomposites are applied as adsorbents and filtering membranes to remove chemical impurities from aqueous media Adeol and Nomngongo (2022). The synthesis of clay polymer nano- composites (CPNs), is becoming famous because it combines the beneficial attributes of both clay minerals and polymers in a single adsorbent. CPNs possesses numerous applications in various industries, including water decontamination Awasthi et al. (2019).
Nanostructured sorbents also possess great potential for the removal of contaminants from the wastewater. The research is now focused on the development of polymer nanocomposites (PNCs), which have increased their efficiency in pollution remediation. Many chemical contaminants, such as heavy metals, hydrocarbons and dyes, have been removed from polluted water using polymer nanocomposites. Both natural as well as synthetic polymers can be utilized for the development of PNCs. Natural polymers include wool, cellulose, proteins and silk. Synthetic polymers consist of polyester, polyethylene, epoxy and teflon.
Nanotechnology have shown great promise in the laboratory studies however its commercialization is the real task. Full scale commercialization of nanoparticles requires significant amount of research
11. Conclusion
Nanotechnology for water and wastewater treatment is gaining momentum globally. The unique properties of nanomaterials and their convergence with current treatment technologies present great opportunities to revolutionize water and wastewater treatment. Although many nanotechnologies highlighted in this review are still in the laboratory research stage, some have made their way to pilot testing or even commercialization. Thus commercialization of these technologies is real task and full scaling requires significant amount of research.
Despite of superior performance, the adoption of nanoparticles for water disinfection must overcome the technical hurdles cost effectiveness and potential environment and human risk. The cost effectiveness can be improved by retaining and reusing the nanoparticles or using low purity nanomaterials with equivalent efficiency Qu et al. (2013). The existing infrastructure must be compatible with the new technologies such as nanotechnology.
Among them, three categories show most promise in full scale application in the near future based on their stages in research and development, commercial availability and cost of nanomaterials involved, and compatibility with the existing infrastructure: nano-adsorbents, nano-technology enabled membranes, and nano-photocatalysts Anjum et al. (2019). The use of nanomaterials for disinfection of water processes or waste-water treatment is restricted by the limited performance of various nanotechnologies in testing the real natural or waste waters. Future research needs more realistic conditions that can assess the application of nanomaterials at commercial scale. Another aspect of nanotechnology-based applications for water disinfection is limited due to limited research on long term effects of these nanoparticles on the flora and fauna present in water Vukoje et al. (2014).
The challenges faced by water/wastewater treatment nanotechnologies are important, but many of these challenges are perhaps only temporary, including technical hurdles, high cost, and potential environmental and human risk Kumari et al. (2019). To overcome these barriers, collaboration between research institutions, industry, government, and other stakeholders is essential. It is our belief that advancing nanotechnology by carefully steering its direction while avoiding unintended consequences can continuously provide robust solutions tour water/wastewater treatment challenges, both incremental and revolutionary Hossain and Hossain (2021).
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Abdulrahman, J. N. (2022). Isolation and Identification of Salmonella spp. from Chicken Meat in Kurdistan Region. Sciences, 20(1), 111–119. https://doi.org/10.32649/ajas.2022.175492
Abid, N., Khan, A. M., Shujait, S., Chaudhary, K., Ikram, M., Imran, M., Haider, J., Khan, M., Khan, Q., & Maqbool, M. (2022). Synthesis of Nanomaterials Using Various Top-Down and Bottom-Up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Advances in Colloid and Interface Science, 300, 102597. https://doi.org/10.1016/j.cis.2021.102597
Abraham, W. R. (2011). Megacities as Sources for Pathogenic Bacteria in Rivers and Their Fate Downstream. International Journal of Microbiology, 2011, 798292. https://doi.org/10.1155/2011/798292
Adeleye, A. S., Wang, X., Wang, F., Hao, R., Song, W., & Li, Y. (2018). Photoreactivity of Graphene Oxide in Aqueous System: Reactive Oxygen Species Formation and Bisphenol A Degradation. Chemosphere, 195, 344–350. https://doi.org/10.1016/j.chemosphere.2017.12.095
Adeola, A. O., & Nomngongo, P. N. (2022). Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers, 14(12), 2462. https://doi.org/10.3390/polym14122462
Agnihotri, S., Mukherji, S., & Mukherji, S. (2014). Size-Controlled Silver Nanoparticles Synthesized over the Range 5–100 nm Using the Same Protocol and their Antibacterial Efficacy. RSC Advances, 4(8), 3974–3983. https://doi.org/10.1039/C3RA44507K
Ahmad, J., Naeem, S., Ahmad, M., Usman, A. R., & Al-Wabel, M. I. (2019). A Critical Review on Organic Micropollutants Contamination in Wastewater and Removal Through Carbon Nanotubes. Journal of Environmental Management, 246, 214–228. https://doi.org/10.1016/j.jenvman.2019.05.152
Akhavan, O., Ghaderi, E., & Esfandiar, A. (2011). Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by Near-Infrared Irradiation. The Journal of Physical Chemistry B, 115(19), 6279–6288. https://doi.org/10.1021/jp200686k
Akhavan, O., & Ghaderi, E. (2010). Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano, 4(10), 5731–5736. https://doi.org/10.1021/nn101390x
Akhil, K., Jayakumar, J., Gayathri, G., & Khan, S. S. (2016). Effect of Various Capping Agents on Photocatalytic, Antibacterial and Antibiofilm Activities of ZnO Nanoparticles. Journal of Photochemistry and Photobiology B: Biology, 160, 32–42. https://doi.org/10.1016/j.jphotobiol.2016.03.015
Alekseeva, O. V., Bagrovskaya, N. A., & Noskov, A. V. (n.d.). Polystyrene Film Composites Filled with Fullerenes (Doctoral Dissertation, Sumy State University).
Ali, I., Peng, C., Khan, Z. M., Naz, I., Sultan, M., Ali, M., Abbasi, I. A., Islam, T., & Ye, T. (2019). Overview of Microbes Based Fabricated Biogenic Nanoparticles for Water and Wastewater Treatment. Journal of Environmental Management, 230, 128–150. https://doi.org/10.1016/j.jenvman.2018.09.073
Anirudhan, T. S., & Deepa, J. R. (2017). Nano-Zinc Oxide Incorporated Graphene Oxide/Nanocellulose Composite for the Adsorption and Photocatalytic Degradation of Ciprofloxacin Hydrochloride from Aqueous Solutions. Journal of Colloid and Interface Science, 490, 343–356. https://doi.org/10.1016/j.jcis.2016.11.042
Anjum, M., Miandad, R., Waqas, M., Gehany, F., & Barakat, M. A. (2019). Remediation of Wastewater using Various Nano-Materials. Arabian Journal of Chemistry, 12(8), 4897–4919. https://doi.org/10.1016/j.arabjc.2016.10.004
Aoki, Y., Kunitake, T., & Nakao, A. (2005). Sol–Gel Fabrication of Dielectric HfO2 Nano-Films ; Formation of Uniform, Void-Free Layers and their Superior Electrical Properties. Chemistry of Materials, 17(2), 450–458. https://doi.org/10.1021/cm048971r
Aqel, A., Abou El-Nour, K. M., Ammar, R. A., & Al-Warthan, A. (2012). Carbon Nanotubes, Science and Technology Part (I) Structure, Synthesis and Characterisation. Arabian Journal of Chemistry, 5(1), 1–23. https://doi.org/10.1016/j.arabjc.2010.08.022
Arshad, M. U., Ghani, M. U., Ullah, A., Güngör, A., & Zaman, M. (2019). Thermodynamic Analysis and Optimization of Double Effect Absorption Refrigeration System using Genetic Algorithm. Energy Conversion and Management, 192, 292–307. https://doi.org/10.1016/j.enconman.2019.03.083
Aslan, S., Deneufchatel, M., Hashmi, S., Li, N., Pfefferle, L. D., Elimelech, M., Pauthe, E., & Van Tassel, P. R. (2012). Carbon Nanotube-based Antimicrobial Biomaterials Formed via Layer-by-Layer Assembly with PolypeptIdes. Journal of Colloid and Interface Science, 388(1), 268–273. https://doi.org/10.1016/j.jcis.2012.08.025
Astefanei, A., Núñez, O., & Galceran, M. T. (2015). Characterisation and Determination of Fullerenes: A Critical Review. Analytica Chimica Acta, 882, 1–21. https://doi.org/10.1016/j.aca.2015.03.025
Awasthi, A., Jadhao, P., & Kumari, K. (2019). Clay Nano-Adsorbent: Structures, Applications and Mechanism for Water Treatment. SN Applied Sciences, 1, 1–21. https://doi.org/10.1007/s42452-019-0858-9
Ayyaru, S., Dinh, T. T., & Ahn, Y. H. (2020). Enhanced Antifouling Performance of PVDF Ultrafiltration Membrane by Blending Zinc Oxide With Support of Graphene Oxide Nanoparticle. Chemosphere, 241, 125068. https://doi.org/10.1016/j.chemosphere.2019.125068
Azizi-Lalabadi, M., Hashemi, H., Feng, J., & Jafari, S. M. (2020). Carbon Nanomaterials Against Pathogens; the Antimicrobial Activity of Carbon Nanotubes, Graphene/Graphene Oxide, Fullerenes, and their Nanocomposites. Advances in Colloid and Interface Science, 284, 102250. https://doi.org/10.1016/j.cis.2020.102250
Babaei-Ghazvini, A., Acharya, B., & Korber, D. R. (2021). Antimicrobial Biodegradable Food Packaging Based on Chitosan and Metal/Metal-Oxide Bio-Nanocomposites: A Review. Polymers, 13(16), 2790. https://doi.org/10.3390/polym13162790
Bag, S. S., Bora, A., & Golder, A. K. (2021). Turning wastes into value‐added materials: Polystyrene Nanocomposites (PS–AgNPs) from Waste Thermocol and Green Synthesized Silver Nanoparticles for Water Disinfection Application. Polymer Composites, 42(11), 6094–6105. https://doi.org/10.1002/pc.26287
Ballatore, M. B., Pérez, M. E., Santamarina, S. C., Durantini, J. E., Milanesio, M. E., & Durantini, E. N. (2022). Photodynamic Polymers Constituted by Porphyrin Units as Antibacterial Materials. Photochem, 2(4), 891–904. https://doi.org/10.3390/photochem2040057
Barbosa, M. O., Moreira, N. F., Ribeiro, A. R., Pereira, M. F., & Silva, A. M. (2016). Occurrence and Removal of Organic Micropollutants: An Overview of the Watch List of EU Decision 2015/495. Water Research, 94, 257–279. https://doi.org/10.1016/j.watres.2016.02.047
Bellar, T. A., Lichtenberg, J. J., & Kroner, R. C. (1974). The occurrence of Organohalides in Chlorinated Drinking Waters. Journal–American Water Works Association, 66(12), 703–706. https://doi.org/10.1002/j.1551-8833.1974.tb02129.x
Benigno, E., Lorente, M. A., Olmos, D., González‐Gaitano, G., & González‐Benito, J. (2019). Nanocomposites Based on Low Density Polyethylene Filled with Carbon Nanotubes Prepared by High Energy Ball Milling and their Potential Antibacterial Activity. Polymer International, 68(6), 1155–1163. https://doi.org/10.1002/pi.5808
Benstoem, F., Nahrstedt, A., Boehler, M., Knopp, G., Montag, D., Siegrist, H., & Pinnekamp, J. (2017). Performance of Granular Activated Carbon to remove Micropollutants from Municipal Wastewater–A Meta-Analysis of Pilot-and Large-Scale Studies. Chemosphere, 185, 105–118. https://doi.org/10.1016/j.chemosphere.2017.06.118
Bing, W., Sun, H., Yan, Z., Ren, J., & Qu, X. (2016). Programmed Bacteria Death Induced by Carbon Dots With Different Surface Charge. Small, 12(34), 4713–4718. https://doi.org/10.1002/smll.201600294
Bolotin, K. I., Sikes, K. J., Jiang, Z., Klima, M., Fudenberg, G., Hone, J., Kim, P., & Stormer, H. L. (2008). Ultrahigh Electron Mobility in Suspended Graphene. Solid State Communications, 146(9-10), 351–355. https://doi.org/10.1016/j.ssc.2008.02.024
Brandelli, A., Ritter, A. C., & Veras, F. F. (2017). Antimicrobial Activities of Metal Nanoparticles. In Metal Nanoparticles in Pharma (pp. 337–363). https://doi.org/10.1007/978-3-319-63790-7_15
Brown, N. F., Wickham, M. E., Coombes, B. K., & Finlay, B. B. (2006). Crossing the Line: Selection and Evolution of Virulence Traits. PLoS Pathogens, 2(5), e42. https://doi.org/10.1371/journal.ppat.0020042
C Thomas, S., Kumar Mishra, P., & Talegaonkar, S. (2015). Ceramic nanoparticles: Fabrication Methods and Applications in Drug Delivery. Current Pharmaceutical Design, 21(42), 6165–6188. https://doi.org/10.2174/1381612821666151027153246
Cabral, J. P. (2010). Water microbiology. Bacterial Pathogens and Water. International Journal of Environmental Research and Public Health, 7(10), 3657–3703. https://doi.org/10.3390/ijerph7103657
Chauhan, A., Sillu, D., & Agnihotri, S. (2019). Removal of pharmaceutical Contaminants in Wastewater Using Nanomaterials: A Comprehensive Review. Current Drug Metabolism, 20(6), 483–505. https://doi.org/10.2174/1389200220666181127104812
Chen, J., & Li, Y. (2016). The Road to MOF‐Related Functional Materials and Beyond: Desire, Design, Decoration, and Development. The Chemical Record, 16(3), 1456–1476. https://doi.org/10.1002/tcr.201500304
Coker, V. S., Green, M., & Corr, S. A. (2012). Nanoscience: Volume 1: Nanostructures Through Chemistry. Royal Society of Chemistry. https://doi.org/10.1039/9781849734804
Costet, N., Villanueva, C. M., Jaakkola, J. J., Kogevinas, M., Cantor, K. P., King, W. D., Lynch, C. F., Nieuwenhuijsen, M. J., & Cordier, S. (2011). Water Disinfection by-Products and Bladder Cancer : Is There a European Specificity? A Pooled and Meta-Analysis of European Case-Control Studies. Occupational and Environmental Medicine, 68(5), 379–385. https://doi.org/10.1136/oem.2010.062703
da Silva, B. L., Caetano, B. L., Chiari-Andréo, B. G., Pietro, R. C., & Chiavacci, L. A. (2019). Increased Antibacterial Activity of ZnO Nanoparticles: Influence of Size and Surface Modification. Colloids and Surfaces B: Biointerfaces, 177, 440–447. https://doi.org/10.1016/j.colsurfb.2019.02.013
Das, S., Sinha, S., Das, B., Jayabalan, R., Suar, M., Mishra, A., Tamhankar, A. J., Stålsby Lundborg, C., & Tripathy, S. K. (2017). Disinfection of Multidrug Resistant Escherichia Coli by Solar-Photocatalysis using Fe-Doped Zno Nanoparticles. Scientific Reports, 7(1), 1–4. https://doi.org/10.1038/s41598-017-00173-0
de Oliveira, L. R., Rodrigues, T. A., Costa, H. L., & da Silva Jr, W. M. (2022). Scuffing Resistance of Polyalphaolefin (PAO)-based Nanolubricants with Oleic Acid (OA) and Iron Oxide Nanoparticles. Materials Today Communications, 31, 103837. https://doi.org/10.1016/j.mtcomm.2022.103837
Deka, B., Baruah, C., Babu, A., & Kalita, P. (2022). Biological and Non-Conventional Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs): Their Potential Applications. Journal of Nanotechnology and Nanomaterials, 3(2), 79–89. https://doi.org/10.33696/Nanotechnol.3.034
Dolati, M., Tafvizi, F., Salehipour, M., Komeili Movahed, T., & Jafari, P. (2023). Biogenic Copper Oxide Nanoparticles From Bacillus coagulans Induced Reactive Oxygen Species Generation and apoptotic and Anti-Metastatic Activities in Breast Cancer Cells. Scientific Reports, 13(1), 3256. https://doi.org/10.1038/s41598-023-30436-y
Dong, Y. D., Zhang, H., Zhong, G. J., Yao, G., & Lai, B. (2021). Cellulose/Carbon Composites and their Applications in Water Treatment—a review. Chemical Engineering Journal, 405, 126980. https://doi.org/10.1016/j.cej.2020.126980
Dubey, R., Dutta, D., Sarkar, A., & Chattopadhyay, P. (2021). Functionalized Carbon Nanotubes : Synthesis, Properties and Applications in water Purification, Drug Delivery, and Material and Biomedical Sciences. Nanoscale Advances, 3(20), 5722–5744. https://doi.org/10.1039/D1NA00293G
Duri, S., Harkins, A. L., Frazier, A. J., & Tran, C. D. (2017). Composites Containing Fullerenes and Polysaccharides : Green and Facile Synthesis, Biocompatibility, and Antimicrobial Activity. ACS Sustainable Chemistry & Engineering, 5(6), 5408–5417. https://doi.org/10.1021/acssuschemeng.7b00715
Elliott, J. A., Shibuta, Y., Amara, H., Bichara, C., & Neyts, E. C. (2013). Atomistic Modelling of CVD Synthesis of Carbon Nanotubes and Graphene. Nanoscale, 5(15), 6662–6676. https://doi.org/10.1039/c3nr01925
Elliott, J. A., Shibuta, Y., Amara, H., Bichara, C., & Neyts, E. C. (2013). Phytosynthesis of Cu/RGO Using Euphorbia Cheiradenia Boiss Extract and Study of its Ability in The Reduction of Organic Dyes and 4‐Nitrophenol in Aqueous Medium. IET Nanobiotechnology, 13(2), 202–213. https://doi.org/10.1049/iet-nbt.2018.5175
Fakhri, A., Azad, M., Fatolahi, L., & Tahami, S. (2018). Microwave-Assisted Photocatalysis of Neurotoxin Compounds Using Metal Oxides Quantum Dots/Nanosheets Composites: Photocorrosion Inhibition, Reusability and Antibacterial Activity Studies. Journal of Photochemistry and Photobiology B: Biology, 178, 108–114. https://doi.org/10.1016/j.jphotobiol.2017.10.038
Fan, M., Gong, L., Huang, Y., Wang, D., & Gong, Z. (2018). Facile Preparation of Silver Nanoparticle Decorated Chitosan Cryogels for Point-of-use Water Disinfection. Science of The Total Environment, 613, 1317–1323. https://doi.org/10.1016/j.scitotenv.2017.09.256
Fang, H., Zhang, Q., Nie, X., Chen, B., Xiao, Y., Zhou, Q., Liao, W., & Liang, X. (2017). Occurrence and Elimination of Antibiotic Resistance Genes in a Long-Term Operation Integrated Surface Flow Constructed Wetland. Chemosphere, 173, 99–106. https://doi.org/10.1016/j.chemosphere.2017.01.027
Gavrilescu, M., Demnerová, K., Aamand, J., Agathos, S., & Fava, F. (2015). Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnology, 32(1), 147–156. https://doi.org/10.1016/j.nbt.2014.01.001
Geim, A. K., & Novoselov, K. S. (2007). The rise of graphene. Nature Materials, 6(3), 183–191. https://doi.org/10.1038/nmat1849
Gerbersdorf, S. U., Cimatoribus, C., Class, H., Engesser, K. H., Helbich, S., Hollert, H., Lange, C., Kranert, M., Metzger, J., Nowak, W., & Seiler, T. B. (2015). Anthropogenic Trace Compounds (ATCs) in Aquatic Habitats—Research Needs on Sources, Fate, Detection and Toxicity to Ensure Timely Elimination Strategies and Risk Management. Environment International, 79, 85–105. https://doi.org/10.1016/j.envint.2015.03.011
Godoy-Gallardo, M., Eckhard, U., Delgado, L. M., de Roo Puente, Y. J., Hoyos-Nogués, M., Gil, F. J., & Perez, R. A. (2021). Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From mechanisms to applications. Bioactive Materials, 6(12), 4470–4490. https://doi.org/10.1016/j.bioactmat.2021.04.033
Goldwater, P. N., & Bettelheim, K. A. (2012). Treatment of Enterohemorrhagic Escherichia coli (EHEC) Infection and Hemolytic Uremic Syndrome (HUS). BMC Medicine, 10, 12. https://doi.org/10.1186/1741-7015-10-12
Graham, D. Y. (2014). History of Helicobacter pylori, Duodenal Ulcer, gastric Ulcer and Gastric Cancer. World Journal of Gastroenterology, 20(18), 5191. https://doi.org/10.3748/wjg.v20.i18.5191
Grzelczak, M., Sánchez‐Iglesias, A., Rodríguez‐González, B., Alvarez‐Puebla, R., Pérez‐Juste, J., & Liz‐Marzán, L. M. (2008). Influence of Iodide Ions on the Growth of Gold Nanorods: Tuning Tip Curvature and Surface Plasmon Resonance. Advanced Functional Materials, 18(23), 3780–3786. https://doi.org/10.1002/adfm.200800706
Guan, Z., Ying, S., Ofoegbu, P. C., Clubb, P., Rico, C., He, F., & Hong, J. (2022). Green Synthesis of Nanoparticles: Current Developments and Limitations. Environmental Technology & Innovation, 102336. https://doi.org/10.1016/j.eti.2022.102336
Gudkov, S. V., Burmistrov, D. E., Serov, D. A., Rebezov, M. B., Semenova, A. A., & Lisitsyn, A. B. (2021). A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Frontiers in Physics, 9, 641481. https://doi.org/10.3389/fphy.2021.641481
Gulig, P. A., Bourdage, K. L., & Starks, A. M. (2005). Molecular pathogenesis of Vibrio vulnificus. Journal of Microbiology, 43(spc1), 118–131.
Guo, D., Xie, G., & Luo, J. (2013). Mechanical properties of nanoparticles: Basics and applications. Journal of Physics D: Applied Physics, 47(1), 013001. https://doi.org/10.1088/0022-3727/47/1/013001
Gutiérrez-Cruz, A., Ruiz-Hernández, A. R., Vega-Clemente, J. F., Luna-Gazcón, D. G., & Campos-Delgado, J. (2022). A Review of Top-Down and Bottom-Up Synthesis Methods for the Production of Graphene, Graphene Oxide and Reduced Graphene Oxide. Journal of Materials Science, 57(31), 14543–14578. https://doi.org/10.1007/s10853-022-07514-z
Hajipour, M. J., Fromm, K. M., Ashkarran, A. A., de Aberasturi, D. J., de Larramendi, I. R., Rojo, T., Serpooshan, V., Parak, W. J., & Mahmoudi, M. (2012). Antibacterial Properties of Nanoparticles. Trends in Biotechnology, 30(10), 499–511. https://doi.org/10.1016/j.tibtech.2012.06.004
He, L., Dong, Y., Zheng, Y., Jia, Q., Shan, S., & Zhang, Y. (2019). A Novel Magnetic MIL-101 (Fe)/TiO2 Composite for Photo Degradation of Tetracycline Under Solar Light. Journal of Hazardous Materials, 361, 85–94. https://doi.org/10.1016/j.jhazmat.2018.08.079
Hennebel, T., De Corte, S., Verstraete, W., & Boon, N. (2012). Microbial Production and Environmental Applications of Pd Nanoparticles for Treatment of Halogenated Compounds. Current Opinion in Biotechnology, 23(4), 555–561. https://doi.org/10.1016/j.copbio.2012.01.007
Homem, V., & Santos, L. (2011). Degradation and Removal Methods of Antibiotics from Aqueous Matrices: A Review. Journal of Environmental Management, 92(10), 2304–2347. https://doi.org/10.1016/j.jenvman.2011.05.023
Hong, M., Miao, Z., Xu, X., & Zhang, Q. (2020). Magnetic Iron Oxide Nanoparticles Immobilized with Sugar-Containing Poly(Ionic liquid) Brushes for Efficient Trapping and Killing of Bacteria. ACS Applied Bio Materials, 3(6), 3664–3672. https://doi.org/10.1021/acsabm.0c00298
Hopkins, Z. R., & Blaney, L. (2016). An Aggregate Analysis of Personal Care Products in the Environment: Identifying the Distribution of Environmentally-Relevant Concentrations. Environment International, 92, 301–316. https://doi.org/10.1016/j.envint.2016.04.026
Hornyak, G. L., Tibbals, H. F., Dutta, J., & Moore, J. J. (2008). Introduction to Nanoscience and Nanotechnology. CRC Press. https://doi.org/10.1201/9781420047806
Hoseinzadeh, E., Makhdoumi, P., Taha, P., Hossini, H., Stelling, J., & Amjad Kamal, M. (2017). A Review on Nano-Antimicrobials: Metal Nanoparticles, Methods and Mechanisms. Current Drug Metabolism, 18(2), 120–128. https://doi.org/10.2174/1389200217666161201111146
Hossain, M. F., & Hossain, M. F. (2021). Wastewater. In Global Sustainability in Energy, Building, Infrastructure, Transportation, and Water Technology (pp. 237–324). https://doi.org/10.1007/978-3-030-62376-0_13
Hosseini, S. S., Hamadi, A., Foroutan, R., Peighambardoust, S. J., & Ramavandi, B. (2022). Decontamination of Cd²⁺ and Pb²⁺ from Aqueous Solution Using a Magnetic Nanocomposite of Eggshell/Starch/Fe₃O₄. Journal of Water Process Engineering, 48, 102911. https://doi.org/10.1016/j.jwpe.2022.102911
Hu, W., Peng, C., Luo, W., Lv, M., Li, X., Li, D., Huang, Q., & Fan, C. (2010). Graphene-Based Antibacterial Paper. ACS Nano, 4(7), 4317–4323. https://doi.org/10.1021/nn101097v
Huang, Z., Dai, X., Huang, Z., Wang, T., Cui, L., Ye, J., & Wu, P. (2019). Simultaneous and Efficient Photocatalytic Reduction of Cr(VI) and Oxidation of Trace Sulfamethoxazole Under LED Light by rGO@Cu₂O/BiVO₄ pn Heterojunction Composite. Chemosphere, 221, 824–833. https://doi.org/10.1016/j.chemosphere.2019.01.087
Hussein, M. A., El-Shishtawy, R. M., Alamry, K. A., Asiri, A. M., & Mohamed, S. A. (2019). Efficient Water Disinfection Using Hybrid polyaniline/Graphene/Carbon Nanotube Nanocomposites. Environmental Technology, 40(21), 2813–2824. https://doi.org/10.1080/09593330.2018.1466921
Ibrahim, R. K., El-Shafie, A., Hin, L. S., Mohd, N. S., Aljumaily, M. M., Ibraim, S., & AlSaadi, M. A. (2019). A Clean Approach for Functionalized Carbon Nanotubes by Deep Eutectic Solvents and their Performance in the Adsorption of Methyl Orange from Aqueous Solution. Journal of Environmental Management, 235, 521–534. https://doi.org/10.1016/j.jenvman.2019.01.070
Islam, S. E., Hang, D. R., Chen, C. H., & Sharma, K. H. (2018). Facile and Cost-Efficient Synthesis Of Quasi-0D/2D ZnO/MoS₂ Nanocomposites for Highly Enhanced Visible-Light-Driven Photocatalytic Degradation of Organic Pollutants and Antibiotics. Chemistry – A European Journal, 24(37), 9305–9315. https://doi.org/10.1002/chem.201801397
Jiao, L., Wang, Y., Jiang, H. L., & Xu, Q. (2018). Metal-Organic Frameworks as Platforms for Catalytic Applications. Advanced Materials, 30(37), 1703663. https://doi.org/10.1002/adma.201703663
Jin, Y., Zheng, Y., Podkolzin, S. G., & Lee, W. (2020). Band Gap of Reduced Graphene Oxide Tuned by Controlling Functional Groups. Journal of Materials Chemistry C, 8(14), 4885–4894. https://doi.org/10.1039/C9TC07063J
Kang, S., Pinault, M., Pfefferle, L. D., & Elimelech, M. (2007). Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir, 23(17), 8670–8673. https://doi.org/10.1021/la701067r
Karim, S., Bae, S., Greenwood, D., Hanna, K., & Singhal, N. (2017). Degradation of 17α-Ethinylestradiol by Nano Zero Valent Iron Under Different pH and Dissolved Oxygen Levels. Water Research, 125, 32–41. https://doi.org/10.1016/j.watres.2017.08.029
Kefeni, K. K., Mamba, B. B., & Msagati, T. A. (2017). Application of Spinel Ferrite Nanoparticles in Water and Wastewater treatment: A Review. Separation and Purification Technology, 188, 399–422. https://doi.org/10.1016/j.seppur.2017.07.015
Kessler, A., Hedberg, J., Blomberg, E., & Odnevall, I. (2022). Reactive Oxygen Species formed by Metal and Metal Oxide Nanoparticles in Physiological Media-a Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials, 12(11), 1922. https://doi.org/10.3390/nano12111922
Khan, A. A., Khan, A., Rahman, M. M., Asiri, A. M., & Oves, M. (2016). Lead Sensors Development and Antimicrobial Activities Based on Graphene Oxide/Carbon Nanotube/Poly(O-toluidine) Nanocomposite. International Journal of Biological Macromolecules, 89, 198–205. https://doi.org/10.1016/j.ijbiomac.2016.04.064
Khan, I., Saeed, K., & Khan, I. (2019). Nanoparticles: Properties, Applications and Toxicities. Arabian Journal of Chemistry, 12(7), 908–931. https://doi.org/10.1016/j.arabjc.2017.05.011
Khan, S., & Hossain, M. K. (2022). Classification and Properties of Nanoparticles. In Nanoparticle-Based Polymer Composites (pp. 15–54). Woodhead Publishing. https://doi.org/10.1016/B978-0-12-824272-8.00009-9
Koeppenkastrop, D., & De Carlo, E. H. (1993). Uptake of Rare Earth Elements from Solution by Metal Oxides. Environmental Science & Technology, 27(9), 1796–1802. https://doi.org/10.1021/es00046a006
Kooij, D. (2000). Biological stability: A Multidimensional Quality Aspect of Treated Water. In Environmental Challenges 2000 (pp. 25–34). Springer. https://doi.org/10.1007/978-94-011-4369-1_3
Kumar, P., Srivastava, S., Banerjee, A., & Banerjee, S. (2022). Prevalence and Predictors of Water-Borne Diseases Among Elderly People in India: Evidence from Longitudinal Ageing Study in India, 2017–18. BMC Public Health, 22, 993. https://doi.org/10.1186/s12889-022-13376-6
Kumar, S., Ahlawat, W., Bhanjana, G., Heydarifard, S., Nazhad, M. M., & Dilbaghi, N. (2014). Nanotechnology-Based Water Treatment Strategies. Journal of Nanoscience and Nanotechnology, 14(2), 1838–1858. https://doi.org/10.1166/jnn.2014.9050
Kumar, S., Ye, F., Dobretsov, S., & Dutta, J. (2021). Nanocoating is a New way for Biofouling Prevention. Frontiers in Nanotechnology, 90. https://doi.org/10.3389/fnano.2021.771098
Kumar, V., Katyal, D., & Nayak, S. (2020). Removal of Heavy Metals and Radionuclides from Water Using Nanomaterials: Current Scenario and Future Prospects. Environmental Science and Pollution Research, 27, 41199–41224. https://doi.org/10.1007/s11356-020-10348-4
Kumari, M., & Gupta, S. K. (2022). Occurrence and Exposure to Trihalomethanes in Drinking Water : A Systematic Review and Meta-Analysis. Exposure and Health, 14(4), 915–939. https://doi.org/10.1007/s12403-022-00467-3
Kumari, P., Alam, M., & Siddiqi, W. A. (2019). Usage of Nanoparticles as Adsorbents for Wastewater Treatment: An Emerging Trend. Sustainable Materials and Technologies, 22, e00128. https://doi.org/10.1016/j.susmat.2019.e00128
Kurosawa, T., Okamoto, T., Yamashita, Y., Kumagai, S., Watanabe, S., & Takeya, J. (2021). Strong and Atmospherically Stable Dicationic Oxidative Dopant. Advanced Science, 8(24), 2101998. https://doi.org/10.1002/advs.202101998
Lee, K. J., Lee, J. H., Jeoung, S., & Moon, H. R. (2017). Transformation of Metal-Organic Frameworks/Coordination Polymers into Functional Nanostructured Materials: Experimental Approaches Based on Mechanistic Insights. Accounts of Chemical Research, 50(11), 2684–2692. https://doi.org/10.1021/acs.accounts.7b00259
Lee, K. M., Lai, C. W., Ngai, K. S., & Juan, J. C. (2016). Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Research, 88, 428–448. https://doi.org/10.1016/j.watres.2015.09.045
Li, G., Zhao, S., Zhang, Y., & Tang, Z. (2018). Metal-Organic Frameworks Encapsulating Active Nanoparticles as Emerging Composites for Catalysis: Recent Progress and Perspectives. Advanced Materials, 30(51), 1800702. https://doi.org/10.1002/adma.201800702
Liu, D., Mao, Y., & Ding, L. (2018). Carbon Nanotubes as Antimicrobial Agents for Water Disinfection and Pathogen Control. Journal of Water and Health, 16(2), 171–180. https://doi.org/10.2166/wh.2018.228
Luo, Y., Guo, W., Ngo, H. H., Nghiem, L. D., Hai, F. I., Zhang, J., Liang, S., & Wang, X. C. (2014). A Review on the Occurrence of Micropollutants in the Aquatic Environment and their fate and removal during wastewater treatment. Science of the Total Environment, 473, 619–641. https://doi.org/10.1016/j.scitotenv.2013.12.065
Ma, X., Chai, Y., Li, P., & Wang, B. (2019). Metal-Organic Framework Films and their Potential Applications in Environmental Pollution control. Accounts of Chemical Research, 52(5), 1461–1470. https://doi.org/10.1021/acs.accounts.9b00113
Mackenzie, C. R., & Livinstone, D. (1968). Salmonellae in Fish and Food. South African Medical Journal, 42(38), 999–1003.
Makabenta, J. M., Nabawy, A., Li, C. H., Schmidt-Malan, S., Patel, R., & Rotello, V. M. (2021). Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nature Reviews Microbiology, 19(1), 23–36. https://doi.org/10.1038/s41579-020-0420-1
Maksimchuk, P. O., Yefimova, S. L., Hubenko, K. O., Omielaieva, V. V., Kavok, N. S., Klochkov, V. K., Sorokin, O. V., & Malyukin, Y. V. (2020). Dark Reactive Oxygen Species Generation in ReVO4 : Eu3+ (Re= Gd, Y) nanoparticles in aqueous solutions. The Journal of Physical Chemistry C, 124(6), 3843–3850. https://doi.org/10.1021/acs.jpcc.9b10143
Maksimova, Y. G. (2019). Microorganisms and carbon nanotubes : Interaction and Applications. Applied Biochemistry and Microbiology, 55(1), 1–12. https://doi.org/10.1134/S0003683819010101
Maurin, M. (2020). Francisella Tularensis, Tularemia and Serological Diagnosis. Frontiers in Cellular and Infection Microbiology, 10, 512090. https://doi.org/10.3389/fcimb.2020.512090
Mbiri, A., Wittstock, G., Taffa, D. H., Gatebe, E., Baya, J., & Wark, M. (2018). Photocatalytic Degradation of the Herbicide Chloridazon on Mesoporous Titania/Zirconia Nanopowders. Environmental Science and Pollution Research, 25(35), 34873–34883. https://doi.org/10.1007/s11356-017-1023-x
Meynell, E. W. (1961). A phage, χ, Which Attacks Motile Bacteria. Microbiology, 25(2), 253–290. https://doi.org/10.1099/00221287-25-2-253
Mishra, L., Paul, K. K., & Jena, S. (2021). Coke Wastewater Treatment Methods : Mini Review. Journal of the Indian Chemical Society, 98(10), 100133. https://doi.org/10.1016/j.jics.2021.100133
Mohammed Sadiq, I., Chandrasekaran, N., & Mukherjee, A. J. (2010). Studies on Effect of TiO2 Nanoparticles on Growth and Membrane Permeability of Escherichia coli, Pseudomonas aeruginosa, and Bacillus subtilis. Current Nanoscience, 6(4), 381–387. https://doi.org/10.2174/157341310791658973
Morrison, C. M., Hogard, S., Pearce, R., Gerrity, D., von Gunten, U., & Wert, E. C. (2022). Ozone Disinfection of Waterborne Pathogens and their Surrogates: A Critical review. Water Research, 221, 118206. https://doi.org/10.1016/j.watres.2022.118206
Motshekga, S. C., Ray, S. S., & Maity, A. (2018). Synthesis and Characterization Of Alginate Beads Encapsulated Zinc Oxide Nanoparticles for Bacteria Disinfection in Water. Journal of Colloid and Interface Science, 512, 686–692. https://doi.org/10.1016/j.jcis.2017.10.098
Mubarak, N. M., Sahu, J. N., Abdullah, E. C., & Jayakumar, N. S. (2016). Rapid Adsorption of Toxic Pb (II) Ions from Aqueous Solution Using Multiwall Carbon Nanotubes Synthesized by Microwave Chemical Vapor Deposition Technique. Journal of Environmental Sciences, 45, 143–155. https://doi.org/10.1016/j.jes.2015.12.025
Muellner, M. G., Wagner, E. D., McCalla, K., Richardson, S. D., Woo, Y. T., & Plewa, M. J. (2007). Haloacetonitriles vs. Regulated Haloacetic acids: Are Nitrogen-Containing DBPs More Toxic? Environmental Science & Technology, 41(2), 645–651. https://doi.org/10.1021/es0617441
Munnawar, I., Iqbal, S. S., Anwar, M. N., Batool, M., Tariq, S., Faitma, N., … Ahmad, N. M. (2017). Synergistic Effect of Chitosan-zinc Oxide Hybrid Nanoparticles on Antibiofouling and Water Disinfection of Mixed Matrix Polyethersulfone Nanocomposite Membranes. Carbohydrate Polymers, 175, 661–670. https://doi.org/10.1016/j.carbpol.2017.08.036
Neamtu, M., Nadejde, C., Hodoroaba, V. D., Schneider, R. J., Verestiuc, L., & Panne, U. (2018). Functionalized Magnetic Nanoparticles: Synthesis, Characterization, Catalytic Application And Assessment of Toxicity. Scientific Reports, 8(1), 1–12. https://doi.org/10.1038/s41598-018-24721-4
Ng, A., Weerakoon, D., Lim, E., & Padhye, L. P. (2019). Fate of Environmental Pollutants. Water Environment Research, 91(10), 1294–1325. https://doi.org/10.1002/wer.1225
Omran, B., & Baek, K. H. (2022). Graphene-Derived Antibacterial Nanocomposites for Water Disinfection: Current And Future Perspectives. Environmental Pollution, 300, 118836. https://doi.org/10.1016/j.envpol.2022.118836
Ottaviani, D., Leoni, F., Serra, R., Serracca, L., Decastelli, L., Rocchegiani, E., … Carraturo, A. (2012). Nontoxigenic Vibrio Parahaemolyticus Strains Causing Acute Gastroenteritis. Journal of Clinical Microbiology, 50(12), 4141–4143. https://doi.org/10.1128/JCM.01993-12
Pal, D. (2020). Synthesis of metal oxide nanoparticles-A General Overview. Indian Journal of Chemistry-Section A (IJCA), 59(10), 1513–1528. https://doi.org/10.56042/ijca.v59i10.33601
Palza, H. (2015). Antimicrobial Polymers With Metal Nanoparticles. International Journal of Molecular Sciences, 16(1), 2099–2116. https://doi.org/10.3390/ijms16012099
Pan, B., & Xing, B. (2008). Adsorption mechanisms of organic chemicals on carbon nanotubes. Environmental Science & Technology, 42(24), 9005–9013. https://doi.org/10.1021/es801777n
Nkele, A. C., & Ezema, F. I. (2020). Diverse Synthesis And Characterization Techniques Of Nanoparticles. Thin Films.
Noah, N. M., & Ndangili, P. M. (2022). Green Synthesis of Nanomaterials from Sustainable Materials for Biosensors and Drug Delivery. Sensors International, 3, 100166. https://doi.org/10.1016/j.sintl.2022.100166
Nogueira, V., Lopes, I., Freitas, A. C., Rocha-Santos, T. A., Gonçalves, F., Duarte, A. C., & Pereira, R. (2015). Biological Treatment With Fungi of Olive Mill Wastewater Pre-Treated by Photocatalytic Oxidation With Nanomaterials. Ecotoxicology and Environmental Safety, 115, 234–242. https://doi.org/10.1016/j.ecoenv.2015.02.028
Nogueira, V., Lopes, I., Rocha-Santos, T. A., Gonçalves, F., & Pereira, R. (2018). Treatment of Real Industrial Wastewaters Through Nano-TiO2 and Nano-Fe2O3 Photocatalysis: Case Study of Mining and Kraft Pulp Mill Effluents. Environmental Technology, 39(12), 1586–1596. https://doi.org/10.1080/09593330.2017.1334093
Obeid, M. M., & Sun, Q. (2022). Assembling Biphenylene into 3D f Organic Chemicals on Carbon Nanotubes. Environmental Science & Technology, 42(24), 9005–9013. https://doi.org/10.1021/es801777n
Pandiyan, R., Mahalingam, S., & Ahn, Y. H. (2019). Antibacterial and Photocatalytic Activity of Hydrothermally Synthesized SnO2 Doped GO and Cnt Under Visible Light Irradiation. Journal of Photochemistry and Photobiology B: Biology, 191, 18–25. https://doi.org/10.1016/j.jphotobiol.2018.12.007
Park, K., Ali, I., & Kim, J. O. (2018). Photodegradation of Perfluorooctanoic Acid by Graphene Oxide-Deposited TiO2 Nanotube Arrays in Aqueous Phase. Journal of Environmental Management, 218, 333–339. https://doi.org/10.1016/j.jenvman.2018.04.016
Peng, J., Zhang, Y., Zhang, C., Miao, D., Li, J., Liu, H., Wang, L., & Gao, S. (2019). Removal of Triclosan in a Fenton-like System Mediated by Graphene Oxide: Reaction Kinetics and Ecotoxicity Evaluation. Science of the Total Environment, 673, 726–733. https://doi.org/10.1016/j.scitotenv.2019.03.354
Peng, L. M., Zhang, Z., & Qiu, C. (2019). Carbon nanotube digital electronics. Nature Electronics, 2(11), 499–505. https://doi.org/10.1038/s41928-019-0330-2
Perazzoli, S., Michels, C., & Soares, H. M. (2017). Magnetite Nanoparticles Influence the Ammonium-Oxidizing Bacteria Activity During Nitritation Process. Water Science and Technology, 75(1), 165–172. https://doi.org/10.2166/wst.2016.497
Philip, A., & Kumar, A. R. (2022). The Performance Enhancement of Surface Plasmon Resonance Optical Sensors Using Nanomaterials: A Review. Coordination Chemistry Reviews, 458, 214424. https://doi.org/10.1016/j.ccr.2022.214424
Piatkowska, A., Janus, M., Szymanski, K., & Mozia, S. (2021). C-, N- and S-Doped Tio2 Photocatalysts: A Review. Catalysts, 11(1), 144. https://doi.org/10.3390/catal11010144
Prest, E. I., Hammes, F., Van Loosdrecht, M. C., & Vrouwenvelder, J. S. (2016). Biological Stability of Drinking Water : Controlling Factors, Methods, and Challenges. Frontiers in Microbiology, 7, 45. https://doi.org/10.3389/fmicb.2016.00045
Punia, P., Bharti, M. K., Chalia, S., Dhar, R., Ravelo, B., Thakur, P., & Thakur, A. (2021). Recent Advances in Synthesis, Characterization, and Applications of Nanoparticles for Contaminated Water Treatment—a Review. Ceramics International, 47(2), 1526–1550. https://doi.org/10.1016/j.ceramint.2020.09.050
Qu, X., Alvarez, P. J., & Li, Q. (2013). Applications of Nanotechnology in Water and Wastewater Treatment. Water Research, 47(12), 3931–3946. https://doi.org/10.1016/j.watres.2012.09.058
Quirós, J., Boltes, K., Aguado, S., de Villoria, R. G., Vilatela, J. J., & Rosal, R. (2015). Antimicrobial Metal-Organic Frameworks Incorporated into Electrospun Fibers. Chemical Engineering Journal, 262, 189–197. https://doi.org/10.1016/j.cej.2014.09.104
Rai, P. K., Lee, J., Kailasa, S. K., Kwon, E. E., Tsang, Y. F., Ok, Y. S., & Kim, K. H. (2018). A Critical Review of ferrate (VI)-Based Remediation of Soil and Groundwater. Environmental Research, 160, 420–448. https://doi.org/10.1016/j.envres.2017.10.016
Rani, M. (2021). A Review on Contamination of Drinking Water due to Pathogenic Microbes and Water-Borne Illness in Uttarakhand, India. International Research Journal of Environmental Science, 10, 20–23.
Rao, J. P., & Geckeler, K. E. (2011). Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Progress in Polymer Science, 36(7), 887–913. https://doi.org/10.1016/j.progpolymsci.2011.01.001
Rao, S. M., Subburaju, N., Palaniappan, N., & Varadarajan, V. V. (2022). An Unusual Case of Community-Acquired Aeromonas Hydrophila Gastroenteritis Causing Delayed-Onset Obstructive Hydrocephalus in a Child After Posterior Fossa Craniotomy for a Tumor: A Case Report and Review of Literature. Pediatric Infectious Disease, 4(1), 21–23. https://doi.org/10.5005/jp-journals-10081-1309
Rasheed, T., Rizwan, K., Bilal, M., & Iqbal, H. M. (2020). Metal-Organic Framework-Based Engineered Materials—Fundamentals and Applications. Molecules, 25(7), 1598. https://doi.org/10.3390/molecules2507159 8
Ray, S. S., Gusain, R., & Kumar, N. (2020). Carbon Nanomaterial-Based Adsorbents for Water Purification : Fundamentals and Applications. Elsevier. https://doi.org/10.1016/B978-0-12-821959-1.00009-X
Reynoso, E., Durantini, A. M., Solis, C. A., Macor, L. P., Otero, L. A., Gervaldo, M. A., Durantini, E. N., & Heredia, D. A. (2021). Photoactive Antimicrobial Coating based on a PEDOT-Fullerene C60 Polymeric Dyad. RSC Advances, 11(38), 23519–23532. https://doi.org/10.1039/D1RA03417K
Ribeiro, A. I., Dias, A. M., & Zille, A. (2022). Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Applied Nano Materials, 5(3), 3030–3064. https://doi.org/10.1021/acsanm.1c03891
Rojas, S., & Horcajada, P. (2020). Metal-Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chemical Reviews, 120(16), 8378–8415. https://doi.org/10.1021/acs.chemrev.9b00797
Roy, A., Gauri, S. S., Bhattacharya, M., & Bhattacharya, J. (2013). Antimicrobial activity of CaO Nanoparticles. Journal of Biomedical Nanotechnology, 9(9), 1570–1578. https://doi.org/10.1166/jbn.2013.1681
Sahli, C., Moya, S. E., Lomas, J. S., Gravier-Pelletier, C., Briandet, R., & Hémadi, M. (2022). Recent Advances in Nanotechnology for Eradicating Bacterial Biofilm. Theranostics, 12(5), 2383. https://doi.org/10.7150/thno.67296
Saied, E., Salem, S. S., Al-Askar, A. A., Elkady, F. M., Arishi, A. A., & Hashem, A. H. (2022). Mycosynthesis of Hematite (α-Fe2O3) Nanoparticles Using Aspergillus Niger and their Antimicrobial and Photocatalytic Activities. Bioengineering, 9(8), 397. https://doi.org/10.3390/bioengineering9080397
Saifuddin, N., Raziah, A. Z., & Junizah, A. R. (2013). Carbon Nanotubes : A Review on Structure and their Interaction with Proteins. Journal of Chemistry, 2013, 676815. https://doi.org/10.1155/2013/676815
Samuel, M. S., Ravikumar, M., John, J. A., Selvarajan, E., Patel, H., Chander, P. S., Soundarya, J., Vuppala, S., Balaji, R., & Chandrasekar, N. (2022). A Review on Green Synthesis of Nanoparticles and their Diverse Biomedical and Environmental applications. Catalysts, 12(5), 459. https://doi.org/10.3390/catal12050459
Sánchez-López, E., Gomes, D., Esteruelas, G., Bonilla, L., Lopez-Machado, A. L., Galindo, R., Cano, A., Espina, M., Ettcheto, M., Camins, A., & Silva, A. M. (2020). Metal-Based Nanoparticles as Antimicrobial Agents : An Overview. Nanomaterials, 10(2), 292. https://doi.org/10.3390/nano10020292
Sarfraz, S., Javed, A., Mughal, S. S., Bashir, M., Rehman, A., Parveen, S., Khushi, A., & Khan, M. K. (2020). Copper Oxide Nanoparticles: Reactive Oxygen Species Generation and Biomedical Applications. International Journal of Computational and Theoretical Chemistry, 8, 40–46. https://doi.org/10.11648/j.ijctc.20200802.12
Schilling, K., Bradford, B., Castelli, D., Dufour, E., Nash, J. F., Pape, W., Schulte, S., Tooley, I., van den Bosch, J., & Schellauf, F. (2010). Human Safety Review of "Nano" Titanium Dioxide and Zinc Oxide. Photochemical & Photobiological Sciences, 9, 495–509. https://doi.org/10.1039/b9pp00180h
Schurer, R., Schippers, J. C., Kennedy, M. D., Cornelissen, E. R., Salinas-Rodriguez, S. G., Hijnen, W. A., & Van der Wal, A. (2019). Enhancing Biological Stability of Disinfectant-Free Drinking Water by Reducing High Molecular Weight Organic Compounds With Ultrafiltration Posttreatment. Water Research, 164, 114927. https://doi.org/10.1016/j.watres.2019.114927
Seil, J. T., & Webster, T. J. (2012). Antimicrobial Applications of Nanotechnology: Methods and Literature. International Journal of Nanomedicine, 7, 2767–2781. https://doi.org/10.2147/IJN.S24805
Shah, A., Khalil, A. T., Ahmad, K., Iqbal, J., Shah, H., Shinwari, Z. K., & Maaza, M. (2021). Biogenic Nanoparticles: Synthesis, Mechanism, Characterization and Applications. In Biogenic Nanoparticles for Cancer Theranostics (pp. 27–42). Elsevier. https://doi.org/10.1016/B978-0-12-821467-1.00010-0
Sharma, S., Kumar, K., Thakur, N., & Chauhan, M. S. (2020). Ocimum Tenuiflorum Leaf Extract as a Green Mediator for the Synthesis of ZnO Nanocapsules Inactivating Bacterial Pathogens. Chemical Papers, 74(10), 3431–3444. https://doi.org/10.1007/s11696-020-01177-3
Sharma, V. K., & Feng, M. (2019). Water Depollution Using Metal-Organic Frameworks-Catalyzed Advanced Oxidation Processes: A Review. Journal of Hazardous Materials, 372, 3–16. https://doi.org/10.1016/j.jhazmat.2017.09.043
Sharmin, S., Rahaman, M. M., Sarkar, C., Atolani, O., Islam, M. T., & Adeyemi, O. S. (2021). Nanoparticles as AntimicRobial and Antiviral Agents : A Literature-Based Perspective Study. Heliyon, 7(3), e06456. https://doi.org/10.1016/j.heliyon.2021.e06456
Shaterabadi, Z., Nabiyouni, G., Goya, G. F., & Soleymani, M. (2022). The Effect of the Magnetically Dead Layer on the Magnetization and the Magnetic Anisotropy of the Dextran-Coated Magnetite Nanoparticles. Applied Physics A, 128(8), 631. https://doi.org/10.1007/s00339-022-05675-x
Shen, X., Song, J., Kawakami, K., & Ariga, K. (2023). Zero to Zero Nanoarchitectonics with Fullerene: From Molecules to Nanoparticles. Journal of Nanoparticle Research, 25(3), 45. https://doi.org/10.1007/s11051-023-05693-7
Shim, J., Seo, Y. S., Oh, B. T., & Cho, M. (2016). Microbial Inactivation Kinetics and Mechanisms of Carbon-Doped TiO2 (C-TiO2) Under Visible Light. Journal of Hazardous Materials, 306, 133–139. https://doi.org/10.1016/j.jhazmat.2015.12.013
Siddiqi, K. S., Husen, A., & Rao, R. A. (2018). A Review on Biosynthesis of Silver Nanoparticles and their Biocidal Properties. Journal of Nanobiotechnology, 16(1), 1–28. https://doi.org/10.1186/s12951-018-0334-5
Sigmund, W., Yuh, J., Park, H., Maneeratana, V., Pyrgiotakis, G., Daga, A., Taylor, J., & Nino, J. C. (2006). Processing and Structure Relationships in Electrospinning of Ceramic Fiber Systems. Journal of the American Ceramic Society, 89(2), 395–407. https://doi.org/10.1111/j.1551-2916.2005.00807.x
Singh, R., Bhadouria, R., Singh, P., Kumar, A., Pandey, S., & Singh, V. K. (2020). Nanofiltration Technology for Removal of Pathogens Present in Drinking Water. In Waterborne Pathogens (pp. 463–489). Elsevier. https://doi.org/10.1016/B978-0-12-818783-8.00021-9
Sousa, J. C., Ribeiro, A. R., Barbosa, M. O., Pereira, M. F., & Silva, A. M. (2018). A Review on Environmental Monitoring of Water Organic Pollutants Identified by EU Guidelines. Journal of Hazardous Materials, 344, 146–162. https://doi.org/10.1016/j.jhazmat.2017.09.058
Srinivas, K. (2014). Need of Nanotechnology in Education. Science Journal of Education, 2(2), 58–64.
Stueber, D. D., Villanova, J., Aponte, I., Xiao, Z., & Colvin, V. L. (2021). Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics, 13(7), 943. https://doi.org/10.3390/pharmaceutics13070943cs13070943
Sugunan, A., Warad, H. C., Boman, M., & Dutta, J. (2006). Zinc Oxide Nanowires in Chemical Bath on Seeded Substrates: Role of Hexamine. Journal of Sol-Gel Science and Technology, 39(1), 49–56. https://doi.org/10.1007/s10971-006-6969-y
Tahir, M., Zaman, G., & Khan, T. (2019). Prevention Strategies for Mathematical Model MERS-Corona Virus with Stability Analysis and Optimal Control. Journal of Nanoscience and Nanotechnology Applications, 1(1), 1.
Tang, J., & Wang, J. (2018). Metal organic framework with Coordinatively Unsaturated sites as Efficient Fenton-Like Catalyst for Enhanced Degradation of Sulfamethazine. Environmental Science & Technology, 52(9), 5367–5377. https://doi.org/10.1021/acs.est.8b00092
Thota, S., Wang, M., Jeon, S., Maragani, S., Hamblin, M. R., & Chiang, L. Y. (2012). Synthesis and Characterization of Positively Charged Pentacationic [60] fullerene monoadducts for antimicrobial photodynamic inactivation. Molecules, 17(5), 5225–5243. https://doi.org/10.3390/molecules17055225
Tony, A., El-Geuindi, M., Hussein, S. M., & Elwahab, M. Z. (2016). Degradation of an Organophosphorus Insecticide (chlorpyrifos) in Simulated Wastewater Using Advanced Oxidation processes and chemical oxidation. Applied Science Report, 15, 63–73. https://doi.org/10.15192/PSCP.ASR.2016.15.2.6373
Trawiński, J., & Skibiński, R. (2019). Multivariate Comparison of Photocatalytic Properties of Thirteen Nanostructured Metal Oxides for Water Purification. Journal of Environmental Science and Health, Part A, 54(9), 851–864. https://doi.org/10.1080/10934529.2019.1598169
Tsenter, I., Garkusheva, N., Matafonova, G., & Batoev, V. (2022). A Novel Water Disinfection Method Based on Dual-Wavelength UV Radiation of KrCl (222 nm) and XeBr (282 nm) Excilamps. Journal of Environmental Chemical Engineering, 10(3), 107537. https://doi.org/10.1016/j.jece.2022.107537
Uchiya, K. I., Takahashi, H., Nakagawa, T., Yagi, T., Moriyama, M., Inagaki, T., Ichikawa, K., Nikai, T., & Ogawa, K. (2015). Characterization of a Novel Plasmid, pMAH135, from Mycobacterium avium subsp. Hominissuis. PLOS ONE, 10(2), e0117797. https://doi.org/10.1371/journal.pone.0117797
Vavourakis, C. D., Heijnen, L., Peters, M. C., Marang, L., Ketelaars, H. A., & Hijnen, W. A. (2020). Spatial and Temporal Dynamics in Attached and Suspended Bacterial Communities in Three Drinking Water Distribution Systems With variable biological stability. Environmental Science & Technology, 54(22), 14535–14546. https://doi.org/10.1021/acs.est.0c04532
Vinayagasundaram, C., Nesaraj, A. S., & Sivaranjana, P. (2023). Overview on multicomponent ceramic Composite Materials Used for Efficient Photocatalysis—An Update. Journal of the Indian Chemical Society, 100908. https://doi.org/10.1016/j.jics.2023.100908
Von Freymann, G., Ledermann, A., Thiel, M., Staude, I., Essig, S., Busch, K., & Wegener, M. (2010). Three-Dimensional Nanostructures for Photonics. Advanced Functional Materials, 20(7), 1038–1052. https://doi.org/10.1002/adfm.200901838
Vukoje, I. D., Džunuzović, E. S., Vodnik, V. V., Dimitrijević, S., Ahrenkiel, S. P., & Nedeljković, J. M. (2014). Synthesis, Characterization, and Antimicrobial Activity of Poly (GMA-co-EGDMA) Polymer Decorated with Silver Nanoparticles. Journal of Materials Science, 49, 6838–6844. https://doi.org/10.1007/s10853-014-8386-x
Wanda, E. M., Nyoni, H., Mamba, B. B., & Msagati, T. A. (2017). Occurrence of Emerging Micropollutants in Water Systems in Gauteng, Mpumalanga, and North West Provinces, South Africa. International Journal of Environmental Research and Public Health, 14(1), 79. https://doi.org/10.3390/ijerph14010079
Wang, C., Xue, Y., Wang, P., & Ao, Y. (2018). Effects of water Environmental Factors on the Photocatalytic Degradation of Sulfamethoxazole by AgI/UiO-66 Composite Under Visible Light Irradiation. Journal of Alloys and Compounds, 748, 314–322. https://doi.org/10.1016/j.jallcom.2018.03.129
Wang, H., Yuan, X., Wu, Y., Zeng, G., Dong, H., Chen, X., Leng, L., Wu, Z., Peng, L., & Wu, Y. (2016). In Situ Synthesis of In2S3@MIL-125 (Ti) Core-Shell Microparticle for the Removal of Tetracycline from Wastewater by Integrated Adsorption and Visible-Light-Driven Photocatalysis. Applied Catalysis B: Environmental, 186, 19–29. https://doi.org/10.1016/j.apcatb.2015.12.041
Wang, J., Svoboda, L., Němečková, Z., Sgarzi, M., Henych, J., Licciardello, N., & Cuniberti, G. (2021). Enhanced Visible-Light Photodegradation of Fluoroquinolone-Based Antibiotics and E. coli Growth Inhibition using Ag-TiO2 Nanoparticles. RSC Advances, 11(23), 13980–13991. https://doi.org/10.1039/D0RA10403E
Wang, Y., Chen, G., Weng, H., Wang, L., Chen, J., Cheng, S., Zhang, P., Wang, M., Ge, X., Chen, H., & Huang, W. (2021). Carbon-Doped Boron Nitride Nanosheets with Adjustable Band Structure for Efficient Photocatalytic U(VI) Reduction under Visible Light. Chemical Engineering Journal, 410, 128280. https://doi.org/10.1016/j.cej.2020.128280
Wols, B. A., & Hofman-Caris, C. H. (2012). Review of Photochemical Reaction Constants of Organic Micropollutants Required for UV Advanced Oxidation Processes in Water. Water Research, 46(9), 2815–2827. https://doi.org/10.1016/j.watres.2012.03.036
Wu, G. F., Zhu, J., Weng, G. J., Li, J. J., & Zhao, J. W. (2021). Heterodimers of Metal Nanoparticles: Synthesis, Properties, and Biological Applications. Microchimica Acta, 188(10), 1–21. https://doi.org/10.1007/s00604-021-05002-w
Xia, L., Xu, M., Cheng, G., Yang, L., Guo, Y., Li, D., Fang, D., Zhang, Q., & Liu, H. (2018). Facile Construction of Ag Nanoparticles Encapsulated into Carbon Nanotubes With Robust Antibacterial Activity. Carbon, 130, 775–781. https://doi.org/10.1016/j.carbon.2018.01.073
Xiang, W., Zhang, Y., Lin, H., & Liu, C. J. (2017). Nanoparticle/metal-Organic Framework Composites for Catalytic Applications : Current Status and Perspective. Molecules, 22(12), 2103. https://doi.org/10.3390/molecules22122103
Xie, Y. Y., Hu, X. H., Zhang, Y. W., Wahid, F., Chu, L. Q., Jia, S. R., & Zhong, C. (2020). Development and Antibacterial Activities of Bacterial Cellulose/Graphene Oxide-CuO Nanocomposite Films. Carbohydrate Polymers, 229, 115456. https://doi.org/10.1016/j.carbpol.2019.115456
Yadav, N., Tyagi, M., Wadhwa, S., Mathur, A., & Narang, J. (2020). Few Biomedical Applications of Carbon Nanotubes. In Methods in Enzymology (Vol. 630, pp. 347–363). Academic Press. https://doi.org/10.1016/bs.mie.2019.11.005
Yang, K., & Xing, B. (2010). Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and its Application. Chemical Reviews, 110(10), 5989–6008. https://doi.org/10.1021/cr100059s
Yang, L., Huang, J. N., Ji, W., & Mao, M. (2020). Investigations of a New Combined Application of Nanofluids in Heat Recovery and Air Purification. Powder Technology, 360, 956–966. https://doi.org/10.1016/j.powtec.2019.10.053
Yin, R., Chen, Y., He, S., Li, W., Zeng, L., Guo, W., & Zhu, M. (2020). In Situ Photoreduction Of Structural Fe(III) in a Metal-Organic Framework for Peroxydisulfate Activation and Efficient Removal of Antibiotics in Real Wastewater. Journal of Hazardous Materials, 388, 121996. https://doi.org/10.1016/j.jhazmat.2019.121996
Yonogi, S., Matsuda, S., Kawai, T., Yoda, T., Harada, T., Kumeda, Y., Gotoh, K., Hiyoshi, H., Nakamura, S., Kodama, T., & Iida, T. (2014). BEC, a Novel Enterotoxin of Clostridium Perfringens Found in Human Clinical Isolates from Acute Gastroenteritis Outbreaks. Infection and Immunity, 82(6), 2390–2399. https://doi.org/10.1128/IAI.01759-14
Yu, R., Wang, H., Wang, R., Zhao, P., Chen, Y., Liu, G., & Liao, X. (2022). Polyphenol Modified Natural Collagen Fibrous Network Towards Sustainable and Antibacterial Microfiltration Membrane for Efficient Water Disinfection. Water Research, 218, 118469. https://doi.org/10.1016/j.watres.2022.118469
Yu, R., Zhu, R., Jiang, J., Liang, R., Liu, X., & Liu, G. (2021). Mussel-Inspired Surface Functionalization of Polyamide Microfiltration Membrane with Zwitterionic Silver Nanoparticles for Efficient Anti-Biofouling Water Disinfection. Journal of Colloid and Interface Science, 598, 302–313. https://doi.org/10.1016/j.jcis.2021.04.040
Yu, Y., Zhou, Z., Huang, G., Cheng, H., Han, L., Zhao, S., Chen, Y., & Meng, F. (2022). Purifying Water With Silver Nanoparticles (AgNPs)-Incorporated Membranes: Recent Advancements and Critical Challenges. Water Research, 118901. https://doi.org/10.1016/j.watres.2022.118901
Yuan, S., Feng, L., Wang, K., Pang, J., Bosch, M., Lollar, C., Sun, Y., Qin, J., Yang, X., Zhang, P., & Wang, Q. (2018). Stable Metal-Organic frameworks: Design, Synthesis, and Applications. Advanced Materials, 30(37), 1704303. https://doi.org/10.1002/adma.201704303
Zaharia, M. M., Ghiorghita, C. A., Trofin, M. A., Doroftei, F., Rosca, I., & Mihai, M. (2022). Multifunctional Composites of Zwitterionic Resins and Silver Nanoparticles for Point-of-Demand Antimicrobial Applications. Materials Chemistry And Physics, 275, 125225. https://doi.org/10.1016/j.matchemphys.2021.125225
Zaki, S. A., Eltarahony, M. M., & Abd-El-Haleem, D. A. (2019). Disinfection of Water and Wastewater by Biosynthesized Magnetite and Zerovalent Iron Nanoparticles Via NAP-NAR Enzymes of Proteus Mirabilis 10B. Environmental Science and Pollution Research, 26(23), 23661–23678. https://doi.org/10.1007/s11356-019-05479-2
Zeng, T., Zhang, X., Wang, S., Niu, H., & Cai, Y. (2015). Spatial Confinement of a Co₃O₄ Catalyst in Hollow Metal-Organic Frameworks as a Nanoreactor for Improved Degradation of Organic Pollutants. Environmental Science & Technology, 49(4), 2350–2357. https://doi.org/10.1021/es505014z
Zhang, C. M., Xu, L. M., Xu, P. C., & Wang, X. C. (2016). Elimination of Viruses from Domestic Wastewater: Requirements and Technologies. World Journal of Microbiology and Biotechnology, 32(4), 1–9. https://doi.org/10.1007/s11274-016-2018-3
Zhang, D., Zhao, Y. X., Gao, Y. J., Gao, F. P., Fan, Y. S., Li, X. J., Duan, Z. Y., & Wang, H. (2013a). Anti-Bacterial and in Vivo Tumor Treatment by Reactive Oxygen Species Generated by Magnetic Nanoparticles. Journal of Materials Chemistry B, 1(38), 5100–5107. https://doi.org/10.1039/c3tb20907e
Zhang, H., Deng, X., Ma, Q., Cui, Y., Cheng, X., Xie, M., Li, X., & Cheng, Q. (2018). Fabrication of Silver Decorated Graphene Oxide Composite for Photocatalytic Inactivation of Escherichia coli. Journal of Nanoscience and Nanotechnology, 18(4), 2304–2309. https://doi.org/10.1166/jnn.2018.14533
Zhang, L., Lin, C. Y., Zhang, D., Gong, L., Zhu, Y., Zhao, Z., Xu, Q., Li, H., & Xia, Z. (2019). Guiding Principles for Designing Highly Efficient Metal-Free Carbon Catalysts. Advanced Materials, 31(13), 1805252. https://doi.org/10.1002/adma.201805252
Zhang, W., Li, Y., Niu, J., & Chen, Y. (2013b). Photogeneration of Reactive Oxygen Species On Uncoated Silver, Gold, Nickel, and Silicon Nanoparticles and Their Antibacterial Effects. Langmuir, 29(15), 4647–4651. https://doi.org/10.1021/la400500t
Zhang, X., Gao, B., Fang, J., Zou, W., Dong, L., Cao, C., Zhang, J., Li, Y., & Wang, H. (2019). Chemically Activated Hydrochar as an Effective Adsorbent for Volatile Organic Compounds (VOCs). Chemosphere, 218, 680–686. https://doi.org/10.1016/j.chemosphere.2018.11.144
Zhang, Y., Yang, J. C., Fu, M. L., Yuan, B., & Gupta, K. (2019). One-Step Fabrication of Recycled Ag Nanoparticles/Graphene Aerogel with High Mechanical Property for Disinfection and Catalytic Reduction of 4-Nitrophenol. Environmental Technology, 40(25), 3381–3391. https://doi.org/10.1080/09593330.2018.1473503
Zhao, S. N., Wang, G., Poelman, D., & Van Der Voort, P. (2018). Metal-Organic Frameworks Based Materials for Heterogeneous Photocatalysis. Molecules, 23(11), 2947. https://doi.org/10.3390/molecules23112947
Zheng, B., Fan, J., Chen, B., Qin, X., Wang, J., Wang, F., Deng, R., & Liu, X. (2022). Rare-Earth Doping in Nanostructured Inorganic Materials. Chemical Reviews, 122(6), 5519–5603. https://doi.org/10.1021/acs.chemrev.1c00644
Zhou, J., Chen, Y., Lan, L., Zhang, C., Pan, M., Wang, Y., Han, B., Wang, Z., Jiao, J., & Chen, Q. (2019). A Novel Catalase Mimicking Nanocomposite of Mn (II)-Poly-L-Histidine-Carboxylated multi Walled Carbon Nanotubes and the Application To Hydrogen Peroxide Sensing. Analytical Biochemistry, 567, 51–62. https://doi.org/10.1016/j.ab.2018.12.007
Zielińska, A., Carreiró, F., Oliveira, A. M., Neves, A., Pires, B., Venkatesh, D. N., Durazzo, A., Lucarini, M., Eder, P., Silva, A. M., & Santini, A. (2020). Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules, 25(16), 3731. https://doi.org/10.3390/molecules25163731
World Health Organization. (n.d.). Drinking-Water Quality guidelines. World Health Organization.
Retrieved October 6, 2025.
World Health Organization. (2022). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda (4th ed.). World Health Organization.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.