
Isolation and Identification of Phosphate solubilizing bacteria from waste Dumping ground in Mumbai
Sinal John Tuscano 1![]()
![]() ,
Dr. Nilima Gajbhiye 2
,
Dr. Nilima Gajbhiye 2![]()
![]()
1 Department
Life Science, Ramnarain Ruia Autonomous College, L.N. Road, Matunga, Mumbai,
India
2 HOD,
Department Life Science, Ramnarain Ruia Autonomous College, L.N. Road, Matunga,
Mumbai, India
|
|
ABSTRACT |
||
|
Phosphate
solubilizing bacteria can solubilize insoluble phosphate complexes and
convert them into available forms that can be used by plants for better
growth. Phosphorus in chemical fertilizers gets fixed in the soil and becomes
unavailable for plant growth. It is important to find an alternative
inexpensive and sustainable technology that could provide sufficient
Phosphorus nutrition to plants. An efficient Phosphate solubilizing bacteria
was isolated based on its solubilization zone on Pikovskaya’s agar. The
amount of Phosphate solubilized by the bacterial isolate was 490.0 ug/ml
which was significantly higher as compared to control S. aureus which was
131.0 ug/ml. The release of soluble P significantly correlated with a drop in
pH from 7.00 to 3.85 indicating the acid production mechanism of Phosphate
solubilization. The isolated bacterial strain could also mineralize organic
sources of phosphate. It also showed
potential to solubilize phosphate under stress conditions such as heavy
metals and salt. The Phosphate solubilizing bacteria was identified by
MALDI-TOF sequencing and was shown to belong to the genus Serratia. Therefore, the isolated bacterial strain
shows a good potential to be used as a biofertilizer and provide phosphate
nutrition to the plants sustainably. |
|||
|
Received 03 April
2024 Accepted 05 May 2024 Published 18 May 2024 Corresponding Author Dr.
Nilima Gajbhiye, drneem@yahoo.com DOI 10.29121/granthaalayah.v12.i4.2024.5635 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Phosphate Solubilization, Serratia,
Phosphate Solubilization Index, Heavy Metals, pH, Salt, MALDI-TOF |
|||
1. INTRODUCTION
Soil is one of the most important resources which
comprises abundant microorganisms. These microorganisms play an important role
in various plant growth-promoting activities and cycling of nutrients Singh et al. (2015). Phosphorus (P) is the second major plant growth-limiting nutrient
after Nitrogen (N) that affects plant growth and productivity. Although
Phosphorus is abundant in soils, its update is limited as it readily forms
insoluble complexes with Ca, Al, Mg, Mn, and Fe Wan
et al. (2020), Ben
et al. (2009). To Overcome Phosphorus deficiency, many chemical fertilizers used in
agricultural sectors pose a threat to the environment causing soil pollution
and polluting runoff water bodies. Moreover, the applied phosphate chemical
fertilizers are easily fixed into insoluble forms such as Ca3(PO4)2,
FePO4, AlPO4.etc. Patel
et al. (2008), Park
et al. (2009).
Some microorganisms present in the soil are capable of solubilizing phosphate which acts as a natural biofertilizer leading to plant growth and development Park et al. (2009), Dhurve et al. (2017) Several mechanisms such as the production of organic acids, secretion of siderophores, production of CO2, nitrogen assimilation has been suggested on phosphate solubilization by bacteria Xie et al. (2021). Production of organic acids by bacteria converts insoluble phosphates to soluble forms by chelating the cations bound to phosphate Mardad et al. (2013), Chen et al. (2006). Several phosphorus solubilizing bacteria belonging to different species such as Pseudomonas, Serratia, Burkholderia, Pantoea, Bacillus, Enterobacter, Alcaligenes, and Citrobacter have been identified and studied for their phosphate solubilization potential Henri et al. (2008), Pérez et al. (2007), Banerjee et al. (2010), Gyaneshwar et al. (1999), Pande et al. (2017), Patel et al. (2008). The diversity of these soil microbes varies depending on soil conditions pH, temperature, contamination due to heavy metals, and nutritional content of the soil Ndung’u-Magiroi et al. (2012). The application of phosphate-solubilizing microbes can help improve soil quality and can be a great method of sustainable agriculture Wan et al. (2020).
Phosphate solubilizing microbes have been isolated from different soils including metal-contaminated areas, solid waste compost, acidic soils, saltern sediments, in addition to rhizosphere soil Xie et al. (2021). However, many of these microorganisms are not able to survive in new environments when used as biofertilizers mainly because of the difference in soil characteristics from where the bacteria were isolated Susilowati et al. (2019). Bacterial isolates from harsh or stressful environments such as waste dumping sites can prove to be better adapted to stress conditions. Therefore, the isolation of bacteria from stressful environments can prove useful as it increases the chances of bacterial survival in any given soil environment, thus making it a good candidate as a biofertilizer. Bacteria from soils that are drought-prone and are subject to changing PH and temperature should exhibit survival strategies in such soil to sustain their growth, which also forms the basis of the “stress physiology paradigm” Pérez et al. (2007). It was found that phosphate-solubilizing microorganisms do not perform consistently as they show poor adaptability to changes in soil and climatic conditions Dhurve et al. (2017). No study has been reported regarding the ecology of phosphate solubilizing bacteria of Mumbai and its polluted sites. The objective of the current study was to isolate effective phosphate-solubilizing bacteria in polluted sites of the Mumbai region and the scope of their use as potential biofertilizers.
Soil samples were collected from waste dumping grounds in Mumbai. Approximately 500 g of soil at a depth of 0-15 cm was collected in sterile jars, air dried, ground to pass through a sieve (0.2 mm), and stored at 40 C until further analysis.
2.2. Isolation of phosphate
solubilizing bacteria from the soil sample
Isolation of Phosphate solubilizing bacteria was done using readymade dehydrated Pikovskaya’s media obtained from Himedia following the method described by Nautiyal (1999). Ten grams of soil was added to 100 mL of sterile saline and shaken for 2 hours to form a suspension. The suspension was diluted and 0.1 ml of appropriate dilutions were plated on Pikovskaya’s agar plates containing 5g L- of tricalcium Phosphate as the sole source of Phosphorus to selectively screen phosphate solubilizing bacteria. The plates were incubated at 300 C for three days. After 3 days of incubation, the bacterial colonies that developed a clear zone of solubilization were picked and isolated on fresh Pikovskaya’s agar plates to confirm Phosphate solubilization. The resulting colonies were transferred to PVK agar slants and stored at 40 C.
2.3. Phosphate solubilization in
Pikovskaya’s solid agar medium
The bacterial colonies that developed a clear halo zone were investigated for Phosphate solubilization index. This was done by spot-inoculating the isolates in sterilized petri plates containing sterile Pikovsakaya’s agar medium. The plates were incubated at 300 C for 14 days. The phosphate solubilization index was calculated by measuring the zone of solubilization and the colony diameter by using the formula Mardad et al. (2013).
Solubilization index (SI)
= Colony diameter + halo zone diameter![]()
Colony diameter
2.4. Estimation of pH and
phosphate solubilization in Pikovskaya’s broth
The isolates that showed a clear zone of phosphate solubilization were further selected to determine the amount of P solubilized in Pikovskaya’s broth by the method described by Mihalache et al. (2018). One ml overnight grown culture was inoculated in 100 ml of Pikovskaya’s broth containing Tricalcium Phosphate (TCP) as the sole source of Phosphorus and incubated at 300 C for 10 days. Samples were analyzed for the release every 2 days. Due to the presence of suspended insoluble tricalcium phosphate particles in the culture supernatant, it was allowed to sediment at room temperature for 15 min and then centrifuged at a very low speed (350 rpm) for 2 minutes. The residual phosphate in the culture supernatant was dissolved using 1N HCL. The amount of P solubilized was determined by Phosphomolybdate assay Murphy and Riley (1962). Pikovskaya’s broth without bacterial inoculation was used as control and each treatment was prepared in triplicates. The concentration of solubilized P was determined by extrapolating with the standard graph. The initial and final pH of the broth was also determined using the digital pH meter.
2.5. Identification of the
isolated bacteria using matrix-assisted laser desorption/ionization-time of
flight mass spectrometry (MALDI-TOF-MS)
The isolated bacterial strain was identified using matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) by the method described by Othman et al. (2019). Log score and a colour code (green, yellow, and red) were recorded for the MALDI TOF MS profiles of the isolated bacterial strain and a standard reference strain. MALDI TOF MS identification of the bacteria was done at Himedia laboratories, Mumbai.
2.6. Mineralization of organic
phosphate by the bacterial strain
The bacterial strain capable of solubilizing inorganic
phosphate was assessed for its organic phosphate mineralization ability
following the method described by Jorquera
et al. (2008). The source of organic
phosphate used in Pikovskaya’s medium was sodium phytate. One ml overnight grown culture was inoculated in 100 ml of
Pikovskaya’s broth containing sodium phytate as the sole source of Phosphorus
and incubated at 370 C for 7 days. The amount of phosphate released
in the medium was estimated by phosphomolybdate assay.
2.7. Effect of different salt
concentrations on inorganic phosphate solubilization
Pikovskaya’s agar was used to assess the effect of different salt (NaCl) concentrations on the phosphate solubilizing potential of the isolated bacterial strain by the method described by Rfaki et al. (2015). Salt concentrations tested for the isolated bacterial strain were 2.5%, 5%,8%,10% and 20%. Pikovskaya’s agar plates were spot inoculated with 10 µl inoculum of the isolated bacterial strain and incubated at 370 C for 48 hours. Phosphate solubilization was qualitatively assessed by visualizing the zone of solubilization at different salt concentrations.
2.8. Effect of heavy metals on
phosphate solubilization
The effect of three metal salts was studied on phosphate
solubilization by the bacterial strain as described by Singh et al. (2015). Aluminum chloride, zinc sulphate, and lead nitrate were
selected for this study. The concentration of metal salts used in this
experiment was 0-1000 ppm. Stock solutions of the metal salts were prepared in
distilled water and sterilized using a 0.22 um filter under aseptic conditions.
Pikovskaya’s agar was modified using different concentrations of the metal
salts. plates were spot inoculated with 10 µl
inoculum of the isolated bacterial strain and incubated at 370 C for
48 hours. Phosphate solubilization was qualitatively assessed by visualizing
the zone of solubilization at different concentrations of heavy metals.
3. Results
3.1. Isolation of phosphate
solubilizing bacteria from a soil sample
Phosphate solubilizing bacteria was isolated from the soil sample based on the screening strategy that allowed the formation of a halo zone on the Pikovskaya’s agar plates containing tricalcium phosphate as a sole source of Phosphorus. This preliminary investigation allowed the selection of an isolate exhibiting a halo zone as a phosphate-solubilizing bacteria. This was followed by growing the isolated bacteria in Pikovskaya’s liquid media for 7 days which allowed estimation of solubilized phosphate with a decrease in pH of the media.
Table 1
|
Table 1 Isolation of Phosphate Solubilizing Bacteria |
||||
|
Phosphate solubilizing
bacteria |
Phosphate solubilization in
broth (µg/ml) |
Phosphate solubilization
index (mm) |
Initial pH |
Final pH |
|
Bacterial isolate |
490 |
3.0 |
7.0 |
3.42 |
|
Control S. aureus |
131 |
1.45 |
7.0 |
4.34 |
Figure 1
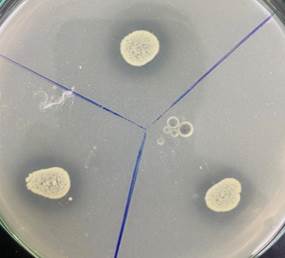
|
Figure 1 Isolated Phosphate Solubilizing Bacteria |
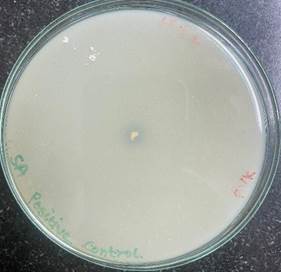
Figure 2
|
Figure 2 Positive Control S. aureus. |
Figure 3

|
Figure 3 Blue Colour Solution in Tube 1 Indicates Positive Phosphate
Solubilization Tube 2-No Phosphate Solubilization in Uninoculated
Control. |
3.2. Organic phosphate
mineralization
Tricalcium Phosphate in Pikovskaya’s broth was replaced with sodium phytate and the medium was assessed for organic phosphate mineralization by the isolated bacterial strain. The isolated bacterial strain showed the potential to mineralize phosphate from sodium phytate which was used as a source of organic phosphate.
|
Phosphate solubilizing
bacteria |
Organic Phosphate
mineralization (µg/ml) |
|
Bacterial isolate |
140.22 |
|
Control S. aureus |
66.15 |
3.3. Effect of salt and heavy metals
on phosphate solubilization
Pikovskaya’s agar with different concentrations of NaCl was used to study phosphate solubilization by the isolated bacterial strain. The bacterial strain showed growth from 0-20% salt concentration. The bacterial isolate showed maximum phosphate solubilization at 2.5% salt concentration. No phosphate solubilization was seen beyond 8% salt concentration.
The isolated bacteria showed phosphate solubilization at different concentrations of metal salts ranging from 0-1000 ppm. The bacterial strain was able to solubilize phosphate even at a very high concentration of the metal salts.
3.4. Identification of phosphate
solubilizing bacteria
The isolated Phosphate solubilizing bacteria was identified at Himedia Laboratories, Mumbai using MALDI-TOF-MS. This advanced method enabled the identification of the bacterial isolate and was considered a fast and accurate method of bacterial identification. The bacterial isolate showed a score value and was identified as Serratia rubidaea. The score value obtained is greater than 9, representing a high degree of precision.
|
Score |
|
|
Sample Serratia rubidaea |
9.569 |
|
Reference Staphylicoccus
aureus ATCC 6538 |
9.707 |
Figure 4
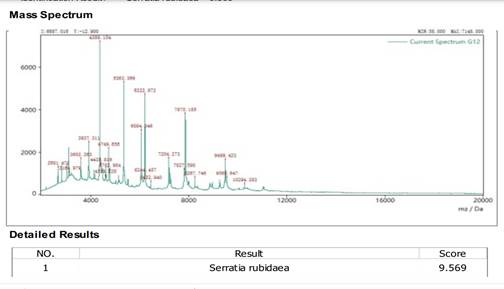
|
Figure 4 MALDI-TOF Sequencing |
4. Discussion
Phosphorus is a limiting
nutrient essential for plant growth. In the past few decades, the use of
biofertilizers over chemical fertilizers has gained a lot of importance. In
this study, preliminary screening of the phosphate-solubilizing bacterial
strain was done using Pikovskaya’s media containing tricalcium phosphate as the
sole phosphorus source. An efficient phosphate-solubilizing bacterial strain
was selected based on its zone of solubilization. This finding is by the
previously reported studies on phosphate solubilizing bacteria Hamdali
(2011), Harpude
et al. (2016), Sharma
et al. (2011). The isolated phosphate solubilizing bacterial strain was screened by
measuring the zone of phosphate solubilization and calculating the
solubilization index. The zone of phosphate solubilization was found to be
much higher as compared to the zone obtained for
positive control S. aureus. The zone
of solubilization obtained was greater than 5mm on Pikovskaya’s agar plates.
Similar findings were reported in previous studies on the isolation of
phosphate-solubilizing bacteria Haouas
et al. (2021). The plate assay was done to select the phosphate-solubilizing bacteria
from a mixed culture plate. Quantitative estimation of the solubilized
phosphate was assessed by the ability of the isolated bacterium to solubilize
phosphate from tricalcium phosphate (TCP) in Pikovskaya’s broth media on the 2nd,
4th, 6th,8th, and 10th day.
Phosphate solubilization increased till the 8th day and was found to
remain constant thereafter. The maximum solubilization observed was 490 µg/ml
as compared to the positive control which was 131 µg/ml. The final pH of the
medium was found to decrease from 7 to 3.42. The result obtained was consistent
with previous studies showing a decrease in the pH of the media by
phosphate-solubilizing bacteria Alikhani
et al. (2006), Zheng
et al. (2018). It was observed that phosphate solubilization was associated with
acidification of the media. A major mechanism associated with a decrease in pH
is the secretion of organic acids by phosphate-solubilizing bacteria that
chelate cations bound to phosphate compounds thereby releasing phosphate in the
media Bolan
et al. (1994), Mardad
et al. (2013), Otani
et al. (1996), Wei et al. (2018). Low molecular weight organic acids such as gluconic acid, succinic
acid, acetic acid, formic acid, etc. are produced due to the oxidation of
glucose by phosphate solubilizing bacteria that involves pqq genes Ben
Farhat et al. (2009).
In most soils, a large amount of phosphate is mostly in the organic form Lidbury et al. (2021). Plants cannot utilize the organic phosphate directly, and hence it needs to be converted to lower phosphate esters which can be available for plant uptake. Charana Walpola (2012). A major organic source of phosphorus includes phytate compounds which are abundant in the soil. Plants cannot uptake phosphate from phytate directly and hence, mineralization of phytate compounds to release phosphate is essential. This occurs by the action of enzymes like phytases and phosphonates Rawat et al. (2021). In this study, organic phosphate mineralization by the bacterium was assessed by using sodium phytate instead of tricalcium phosphate as a source of phosphate in Pikovskaya’s media. The amount of phosphate mineralized by the bacteria was 140.22 µg/ml which was much higher as compared to positive control which was 66.15 µg/ml. Similar studies on the phytate mineralization potential of phosphate solubilizing bacteria were done by Jorquera et al. (2008), Qurban Ali Panhwar (2012) and Singh et al. (2014).
Environmental factors such as the presence of excess salinity and heavy metals affect the growth and phosphate solubilization ability of bacteria. Stress-tolerant bacteria are likely to be found in soil affected by different environmental stresses Nautiyal (1999). The bacterial strain identified as Serratia Rubidaea was isolated from waste dumping ground soil in view to study the phosphate solubilizing ability under stress conditions. In this study, the bacterial strain was exposed to varied concentrations of NaCl ranging from 0-20%. The bacterial strain showed growth on Pikovskaya’s agar plate at all concentrations tested, however, the phosphate solubilization zone was observed up to 8% concentration. Similar studies were done by Son et al. (2006) and Sanjay et al. (2014). The bacterial strain showed better potential to solubilize phosphate as compared to other known phosphate solubilizing bacteria reported by Son et al. (2006) which showed phosphate solubilizing potential at not more than 3% NaCl.
In a similar study, Nakbanpote et al. (2014) demonstrated phosphate solubilization at 8% (w/v) salt concentration. Some bacterial isolates reported by Nautiyal et al. (2000) have also shown phosphate solubilization at 8% and 10% NaCl concentrations.
Phosphate solubilization and metal tolerance ability of plants help in promoting plant growth, particularly in metal-contaminated soils. Phosphate solubilization by the isolated bacteria was assessed in the presence of heavy metal salts such as aluminium chloride, lead nitrate, and zinc sulphate. The concentration of heavy metal salts used was in the range of 0-1000 ppm. The isolated phosphate solubilizing bacterium was able to solubilize phosphate at all tested concentrations.
This bacterial strain was identified as Serratia rubidaea by MALDI-TOF-MS with a score value of 9.56. The score value above 9.5 indicated credible and accurate identification.
5. Conclusion
Phosphorus is an essential element that is involved in a plethora of functional developments during plant growth. A potent phosphate-solubilizing bacterial strain was obtained from a waste dumping ground in Mumbai. The isolate was identified as Serratia Rubidaea by MALDI-TOF sequencing. This study demonstrated the phosphate solubilization potential of the isolated bacterial strain under stress conditions. The isolated strain Serratia Rubidaea showed phosphate solubilizing potential even under stress conditions making it a great candidate to be developed as a biofertilizer for use in soils under harsh environmental.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors are likely to thank the Department of Life Science at Ramnarain Ruia Autonomous College for providing us with the laboratory Equipment and chemicals.
REFERENCES
Alikhani, H. A., Saleh-Rastin, N., & Antoun, H. (2006). Phosphate Solubilization Activity of Rhizobia Native to Iranian Soils. Plant and Soil, 287(1–2), 35–41. https://doi.org/10.1007/s11104-006-9059-6
Banerjee, S., Palit, R., Sengupta, C., & Standing, D. (2010). Stress Induced Phosphate Solubilization by Arthrobacter sp. and Bacillus sp. Isolated from Tomato Rhizosphere. Australian Journal of Crop Science, 4(6), 378–383.
Ben Farhat, M., Farhat, A., Bejar, W., Kammoun, R., Bouchaala, K., Fourati, A., Antoun, H., Bejar, S., & Chouayekh, H. (2009). Characterization of the Mineral Phosphate Solubilizing Activity of Serratia Marcescens CTM 50650 Isolated from the Phosphate Mine of Gafsa. Archives of Microbiology, 191(11), 815–824. https://doi.org/10.1007/s00203-009-0513-8
Bolan, N. S., Naidu, R., Mahimairaja, S., & Baskaran, S. (1994). Influence of Low-Molecular-Weight Organic Acids on the Solubilization of Phosphates. Biology and Fertility of Soils, 18(4), 311–319. https://doi.org/10.1007/BF00570634
Charana Walpola, B. (2012). Prospectus of Phosphate Solubilizing Microorganisms and Phosphorus Availability in Agricultural Soils: A Review. African Journal of Microbiology Research, 6(37), 6600–6605. https://doi.org/10.5897/ajmr12.889
Chen, Y. P., Rekha, P. D., Arun, A. B., Shen, F. T., Lai, W. A., & Young, C. C. (2006). Phosphate Solubilizing Bacteria from Subtropical Soil and Their Tricalcium Phosphate Solubilizing Abilities. Applied Soil Ecology, 34(1), 33–41. https://doi.org/10.1016/j.apsoil.2005.12.002
Dhurve, N. G., Ingle, R. W., Lad, R. S., & Madavi, P. N. (2017). Characterization of Phosphate Solubilizing Bacteria Isolated from Paddy Rhizosphere of Vidarbha Region. 5(6), 24–30.
Gyaneshwar, P., Parekh, L. J., Archana, G., Poole, P. S., Collins, M. D., Hutson, R. A., & Kumar, G. N. (1999). Involvement of a Phosphate Starvation Inducible Glucose Dehydrogenase in Soil Phosphate Solubilization by Enterobacter Asburiae. FEMS Microbiology Letters, 171(2), 223–229. https://doi.org/10.1016/S0378-1097(99)00003-8
Hamdali, H. (2011). Isolation and Characterization of Rock Phosphate Solubilizing Actinobacteria from a Togolese Phosphate Mine. African Journal of Biotechnology, 11(2). https://doi.org/10.5897/ajb11.774
Haouas, A., El Modafar, C., Douira, A., Ibnsouda-Koraichi, S., Filali-Maltouf, A., Moukhli, A., & Amir, S. (2021). Alcaligenes Aquatilis GTE53: Phosphate Solubilising and Bioremediation Bacterium Isolated from New Biotope “Phosphate Sludge Enriched-Compost.” Saudi Journal of Biological Sciences, 28(1), 371–379. https://doi.org/10.1016/j.sjbs.2020.10.015
Harpude, S., Patki, J., & Singh, S. (2016). Isolation and Characterization of Phosphate Solubilising Bacteria from a Discrete Ecological Niche in Navi-Mumbai, Maharashtra, India. 4(Xi), 42–48.
Henri, F., Laurette, N. G. O. N., Annette, D., & John, Q. (2008). Solubilization of Inorganic Phosphates and Plant Growth Promotion by Strains of Pseudomonas Fluorescens Isolated from Acidic Soils of Cameroon. African Journal of Microbiology Research, 2(7), 171–178.
Jorquera, M. A., Hernández, M. T., Rengel, Z., Marschner, P., & De La Luz Mora, M. (2008). Isolation of Culturable Phosphobacteria with Both Phytate-Mineralization and Phosphate-Solubilization Activity from the Rhizosphere of Plants Grown in a Volcanic Soil. Biology and Fertility of Soils, 44(8), 1025–1034. https://doi.org/10.1007/s00374-008-0288-0
Lidbury, I. D. E. A., Borsetto, C., Murphy, A. R. J., Bottrill, A., Jones, A. M. E., Bending, G. D., Hammond, J. P., Chen, Y., Wellington, E. M. H., & Scanlan, D. J. (2021). Niche-Adaptation in Plant-Associated Bacteroidetes Favours Specialisation in Organic Phosphorus Mineralisation. ISME Journal, 15(4), 1040–1055. https://doi.org/10.1038/s41396-020-00829-2
Mardad, I., Serrano, A., & Soukri, A. (2013). Solubilization of Inorganic Phosphate and Production of Organic Acids by Bacteria Isolated from a Moroccan Mineral Phosphate Deposit. African Journal of Microbiology Research, 7(8), 626–635. https://doi.org/10.5897/AJMR12.1431
Mihalache, G., Mihasan, M., Zamfirache, M. M., Stefan, M., & Raus, L. (2018). Phosphate Solubilizing Bacteria from Runner Bean Rhizosphere and Their Mechanism of Action. Romanian Biotechnological Letters, 23(4), 13853–13861. https://doi.org/10.26327/RBL2017.60
Nautiyal, C. S. (1999). An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiology Letters, 170(1), 265–270. https://doi.org/10.1016/S0378-1097(98)00555-2
Ndung’u-Magiroi, K. W., Herrmann, L., Okalebo, J. R., Othieno, C. O., Pypers, P., & Lesueur, D. (2012). Occurrence and Genetic Diversity of Phosphate-Solubilizing Bacteria in Soils of Differing Chemical Characteristics in Kenya. Annals of Microbiology, 62(3), 897–904. https://doi.org/10.1007/s13213-011-0326-2
Otani, T., Ae, N., & Tanaka, H. (1996). Phosphorus (P) Uptake Mechanisms of Crops Grown in Soils with Low P Status: II. Significance of Organic Acids in Root Exudates of Pigeonpea. Soil Science and Plant Nutrition, 42(3), 553–560. https://doi.org/10.1080/00380768.1996.10416324
Othman, M., El-Zamik, F., Hegazy, M., & Salama, A. (2019). Isolation and Identification of Egyptian Strains of Serratia Marcescens Producing Antibacterial and Antioxidant Prodigiosin Pigment. Zagazig Journal of Agricultural Research, 46(5), 1573–1582. https://doi.org/10.21608/zjar.2019.48175
Pande, A., Pandey, P., Mehra, S., Singh, M., & Kaushik, S. (2017). Phenotypic and Genotypic Characterization of Phosphate Solubilizing Bacteria and Their Efficiency on the Growth of Maize. Journal of Genetic Engineering and Biotechnology, 15(2), 379–391. https://doi.org/10.1016/j.jgeb.2017.06.005
Park, K. H., Lee, C. Y., & Son, H. J. (2009). Mechanism of Insoluble Phosphate Solubilization by Pseudomonas Fluorescens RAF15 Isolated from Ginseng Rhizosphere and its Plant Growth-Promoting Activities. Letters in Applied Microbiology, 49(2), 222–228. https://doi.org/10.1111/j.1472-765X.2009.02642.x
Patel, D. K., Archana, G., & Kumar, G. N. (2008). Variation in the Nature of Organic Acid Secretion and Mineral Phosphate Solubilization by Citrobacter sp. DHRSS in the Presence of Different Sugars. Current Microbiology, 56(2), 168–174. https://doi.org/10.1007/s00284-007-9053-0
Pérez, E., Sulbarán, M., Ball, M. M., & Yarzábal, L. A. (2007). Isolation and Characterization of Mineral Phosphate-Solubilizing Bacteria Naturally Colonizing a Limonitic Crust in the South-Eastern Venezuelan Region. Soil Biology and Biochemistry, 39(11), 2905–2914. https://doi.org/10.1016/j.soilbio.2007.06.017
Qurban Ali Panhwar (2012). Isolation and Characterization of Phosphate-Solubilizing Bacteria from Aerobic Rice. African Journal of Biotechnology, 11(11), 2711–2719. https://doi.org/10.5897/ajb10.2218
Rawat, P., Das, S., Shankhdhar, D., & Shankhdhar, S. C. (2021). Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. Journal of Soil Science and Plant Nutrition, 21(1), 49–68. https://doi.org/10.1007/s42729-020-00342-7
Rfaki, A., Nassiri, L., & Ibijbijen, J. (2015). Isolation and Characterization of Phosphate Solubilizing Bacteria from the Rhizosphere of Faba Bean (Vicia faba L.) in Meknes Region, Morocco. British Microbiology Research Journal, 6(5), 247–254. https://doi.org/10.9734/bmrj/2015/14379
Sanjay, A., Purvi, N. P., Meghna, J. V., & G., G. R. (2014). Isolation and Characterization of Endophytic Bacteria Colonizing Halophyte and Other Salt Tolerant Plant Species from Coastal Gujarat. African Journal of Microbiology Research, 8(17), 1779–1788. https://doi.org/10.5897/ajmr2013.5557
Sharma, S., Kumar, V., & Tripathi, R. B. (2011). Isolation of Phosphate Solubilizing Microorganism (PSMs) From Soil. Journal of Microbiology and Biotechnology Research Scholars Research Library J. Microbiol. Biotech. Res, 1(2), 90–95.
Singh, P., Kumar, V., & Agrawal, S. (2014). Evaluation of Phytase Producing Bacteria for Their Plant Growth Promoting Activities. International Journal of Microbiology, 2014. https://doi.org/10.1155/2014/426483
Singh, R., Pathak, B., & Fulekar, M. (2015). Characterization of PGP Traits by Heavy Metals Tolerant Pseudomonas putida and Bacillus safensis Strain Isolated from Rhizospheric Zone of Weed (Phyllanthus urinaria) and its Efficiency in Cd and Pb Removal. Int.J.Curr.Microbiol.App.Sci, 4(7), 954–975.
Son, H. J., Park, G. T., Cha, M. S., & Heo, M. S. (2006). Solubilization of Insoluble Inorganic Phosphates by a Novel Salt- and pH-Tolerant Pantoea Agglomerans R-42 Isolated from Soybean Rhizosphere. Bioresource Technology, 97(2), 204–210. https://doi.org/10.1016/j.biortech.2005.02.021
Susilowati, L. E., Kusumo, B. H., & Arifin, Z. (2019). Screening of the Drought Tolerant Phosphate Solubilizing Bacteria in Dissolving P-Inorganic. Journal of Physics: Conference Series, 1402(5). https://doi.org/10.1088/1742-6596/1402/5/055082
Wan, W., Qin, Y., Wu, H., Zuo, W., He, H., Tan, J., Wang, Y., & He, D. (2020). Isolation and Characterization of Phosphorus Solubilizing Bacteria with Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Frontiers in Microbiology, 11(April), 1–15. https://doi.org/10.3389/fmicb.2020.00752
Wei, Y., Zhao, Y., Shi, M., Cao, Z., Lu, Q., Yang, T., Fan, Y., & Wei, Z. (2018). Effect of Organic Acids Production and Bacterial Community on the Possible Mechanism of Phosphorus Solubilization During Composting with Enriched Phosphate-Solubilizing Bacteria Inoculation. Bioresource Technology, 247, 190–199. https://doi.org/10.1016/j.biortech.2017.09.092
Xie, J., Yan, Z., Wang, G., Xue, W., Li, C., Chen, X., & Chen, D. (2021). A Bacterium Isolated from Soil in a Karst Rocky Desertification Region Has Efficient Phosphate-Solubilizing and Plant Growth-Promoting Ability. Frontiers in Microbiology, 11(February). https://doi.org/10.3389/fmicb.2020.625450
Zheng, B. X., Ibrahim, M., Zhang, D. P., Bi, Q. F., Li, H. Z., Zhou, G. W., Ding, K., Peñuelas, J., Zhu, Y. G., & Yang, X. R. (2018). Identification and Characterization of Inorganic-Phosphate-Solubilizing Bacteria from Agricultural Fields with a Rapid Isolation Method. AMB Express, 8(1). https://doi.org/10.1186/s13568-018-0575-6
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.