
Production of organic acid and solubilization of inorganic phosphate by a bacterium isolated from contaminated soil
Sinal John Tuscano 1![]()
![]() ,
Dr. Nilima Gajbhiye 1
,
Dr. Nilima Gajbhiye 1![]()
1 Department
Life Science, Ramnarain Ruia Autonomous College, L.N. Road, Matunga, Mumbai,
India
2 HOD,
Department Life Science, Ramnarain Ruia Autonomous College, L.N. Road, Matunga,
Mumbai, India
|
|
ABSTRACT |
||
|
Many
agricultural soils have significant phosphorus (P) reserves, much of which
builds up because of frequent P fertilizer applications. However, roughly 95
to 99% of soil phosphorus is found as insoluble phosphates and is therefore
unavailable for plant uptake. The current investigation characterized a
bacterial strain that was obtained from contaminated soil and showed the
ability to solubilize insoluble inorganic phosphates. An efficient
phosphate-solubilizing bacterium was isolated in polluted soil in Mumbai. The
phosphate solubilization index of this isolate was assessed using tribasic
calcium phosphate-supplemented Pikovskaya’s (PVK)
medium. After growing under constant agitation for seven days, the medium pH
decreased from 7.0 to 3.5 units. Based on the colony morphology, microscopic
analysis, and MALDI-TOF sequencing, the bacterial isolate was identified as Klebsiella
pneumoniae. Phosphate solubilization was linked to a pH drop caused by
bacterial growth in a medium with glucose as a carbon source. The secretion
of organic acids by these phosphate-solubilizing bacteria is responsible for
their ability to solubilize inorganic phosphate. GC-MS analysis revealed the
presence of carbamic acid, dodecanoic acid, tetra decanoic acid, and
trifluoroacetic acid in the culture supernatant. The amount of phosphate
solubilized by the bacterium was determined by phosphomolybdate assay and was
found to be 667.0 ug/ml which was much higher than the control bacterium S.
aureus which was 131.0 ug/ml. To the best of our knowledge, this is the
first report mentioning the isolation of phosphate solubilizing bacterium
from polluted soil in Mumbai. |
|||
|
Received 06 December 2023 Accepted 11 January 2024 Published 31 January 2024 Corresponding Author Dr.
Nilima Gajbhiye, nilimalankeshwar@ruiacollege.edu DOI 10.29121/granthaalayah.v12.i1.2024.5470 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Phosphate Solubilization, Organic Acid
Production, Tricalcium Phosphate, Phosphate Solubilization Index |
|||
1. INTRODUCTION
Phosphorus (P), is an important element that is defined as
a macronutrient given that plants need relatively significant amounts of it. Additionally,
it strengthens the straw of the cereal plant and the quality of the harvest,
promotes flower formation and fruit production, and encourages root
development, which is necessary for seed formation, maturity, and the
development of disease resistance. Noorjahan et al. (2019). Although it is in its
fully oxidized state as phosphate, it constantly produces many insoluble
chemical complexes with calcium, iron, and aluminum, which results in the
generation of poorly soluble and inaccessible insoluble phosphate salts Mardad et al. (2013). Thus, during the past century, enormous volumes of phosphate fertilizers
have been used to maximize plant yields. Large levels of P, however, quickly
become immobilized and insoluble, making a significant portion of the phosphate
fertilizer unavailable to plants Wang
et al. (2017).
Soil microorganisms help to preserve the ecological
balance by actively participating in the natural cycles of carbon, nitrogen, sulphur,
and phosphorus Karpagam
& Nagalakshmi (2014). Phosphate
Solubilizing Microorganisms (PSMs) are a broad category of soil bacteria that
are known to solubilize inorganic phosphate Mahadevamurthy et al. (2016). Numerous bacterial genera have been reported to solubilize phosphorus,
including Pseudomonas, Bacillus, Enterobacter, Azotobacter, Agrobacterium,
Achromobacter, Rhizobium, Burkholderia, Flavobacterium, and Micrococcus,
which have been isolated from various soil types Kumar
et al. (2010). Phosphate solubilizing bacteria growth is influenced by various soil
characteristics, including organic matter concentration, phosphorus content,
and physical and chemical characteristics Kadiri
et al. (2013). It is still unclear exactly which genes underlie the bacterial
phenotypic mineral phosphate solubilization in minerals. However, organic acids
generated by glucose dehydrogenase are thought to be the main mechanism behind
phosphate solubilization Chauhan
et al. (2017). More research must be done on this topic as the synthesis of organic acids
by phosphate solubilizing bacteria has not been thoroughly examined.
Phosphate-solubilizing bacteria can have positive
agronomic effects, however, their abundance in the soil is not always
sufficient to compete with other native bacteria. To build sustainable cropping
systems, bacteria must be identified, and characterized, and crop production
and economic-environmental sustainability must be prioritized Janati et al. (2022). Moreover, Waste disposal sites represent an unexplored biological
niche that may support a population of microorganisms with the ability to
solubilize insoluble phosphate. To better understand the solubilization
mechanism, this study aimed to isolate, identify, and characterize
phosphate-solubilizing bacteria (PSB) from this distinctive biotope. This was
achieved by analysing the organic acids that PSB produced.
2. Materials and methods
2.1. Collection of samples
A sample of soil was collected from Mumbai's waste disposal sites. 500 g of soil that had been collected at a depth of 0 to 15 cm was placed in sterile jars, let to air dry, crushed to pass through a 0.2 mm screen, and kept at 40 C until additional analysis was performed.
2.2. Isolation of bacterium solubilizing tricalcium phosphate
The phosphate-solubilizing bacterium was isolated according to the method described by Son et al. (2006). The soil sample from the waste dumping ground was suspended in a sterile saline solution and shaken for six hours. The soil samples were serially diluted and then placed on standard Pikovskaya’s agar medium (glucose 10 g, MgCl2. 6 H2O 5 g, MgSO4. 7 H2O 0.25 g, KCl 0.2 g, and (NH4)2SO4 0.1 g, dissolved in 1 L distilled water). The sole phosphorus source added to the agar medium was 5 g of tribasic calcium phosphate (TCP), which was used to selectively screen for bacteria that can release soluble inorganic phosphate from TCP. Uninoculated plates were used as controls, and the pH was adjusted to 7.0 ± 0.2. Phosphate solubilizing bacteria formed distinct zones surrounding colonies during three days of incubation at 30°C. Clear zone-encircled colonies were selected and streaked onto NBRIP plates. An efficient phosphate-solubilizing bacterium was selected based on its capacity to form a zone of clearance on PVK agar plates.
2.3. Qualitative analysis of phosphate solubilization on PVK agar plates containing bromocresol purple indicator
Phosphate solubilization of the isolated bacterial strain was assessed by a qualitative method using a modified PVK agar medium containing bromocresol purple indicator (0.1g/l) by a method described by Vazquez et al. (2000). The bacterial isolate was spot-inoculated on a PVK bromocresol agar plate and incubated for three days at 30°C. A Color change from purple to yellow indicated positive phosphate solubilization due to a decrease in the pH of the media. By measuring the colony diameter and the zone of solubilization, the phosphate solubilization index was calculated using the formula Pande et al. (2017):
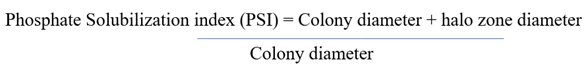
2.4. Phosphate solubilization and pH estimation in Pikovskaya's broth
The quantitative assay for phosphate solubilization by the bacteria was performed by the method described by Rfaki et al. (2015). The bacterium showing a positive result on PVK agar media was assessed for its potential to solubilize inorganic phosphate source (Tricalcium phosphate) in PVK liquid media. 100 ml of PVK broth medium supplemented with (Ca3(PO4)2) and 100 µL of bacteria were inoculated into Erlenmeyer flasks (250 ml) for the quantitative bioassay. Autoclaved uninoculated PVK medium served as control. The flasks were incubated at 30°C on a rotating shaker with 180 rpm. Following seven days of incubation, the growth medium was centrifuged once a day for 20 minutes at 10,000 rpm. After the supernatant was decanted, the pH was measured using a pH meter, and the amount of soluble P released into the solution was measured by the ascorbic acid method. A pH meter was used in each instance to measure the pH of the supernatant.
2.5. Identification of phosphate solubilizing bacteria
The isolated phosphate-solubilizing bacterium was identified using MALDI-TOF-MS at Himedia Laboratories in Mumbai. For both the isolated bacterial strain and a standard reference strain's MALDI TOF MS profiles, a log score, and a colour code (green, yellow, and red) were noted.
2.6. Analysis of Organic acids by gas chromatography-mass spectrometry (GC-MS)
The gas chromatography-mass spectrometry method by Kaur et al. (2018) was used for the analysis of organic acids produced by the phosphate solubilizing bacterium in the broth method. The supernatant of the phosphate solubilizing bacterium grown in PVK medium was taken after centrifuging at 13000 rpm for 15 min. One ml of the oven-dried samples was used to extract the organic acids, and 0.5 ml of 0.5 N HCl and 0.5 ml of methanol were added. Following this, the samples were centrifuged at 12,000 rpm for 10 minutes after being shaken for up to three hours. The mixture was incubated for 6 hours at 60°C in a water bath after the supernatant, 300 μL of methanol, and 100 μL of 50% sulfuric acid were added. The supernatant was cooled to 25 °C before 800 μL of chloroform and 400 μL of distilled water were added, and the mixture was vortexed for one minute. The amount of organic acid in the chloroform was determined by analysing its lower layer. The GC-MS of the samples was done at Dr. P.S. Ramanathan Advanced Instrumentation Centre, Ramnarain Ruia College, Matunga, Mumbai-400019. The organic acids were detected and identified by comparing the peak areas and retention times of their chromatograms with their references in the database.
3. Results
3.1. Isolation of bacterium able to use tricalcium phosphate as a sole source of phosphorus
Soil samples from dumping grounds were collected from a municipal dumping ground in Mumbai to analyze samples that were most affected by the dumping of industrial, agricultural, and domestic wastes. Organisms that can solubilize phosphate produce a distinct zone surrounding their colonies, indicating that the microorganisms are phosphate-solubilizing bacteria. An efficient phosphate-solubilizing bacterium was selected based on its ability to solubilize phosphate and form a zone of solubilization of PVK agar media.
Figure 1
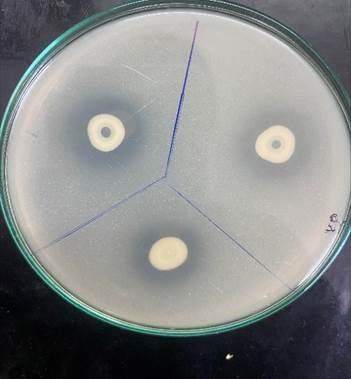
|
Figure 1 Isolated
Phosphate Solubilizing Bacteria |
Figure 2
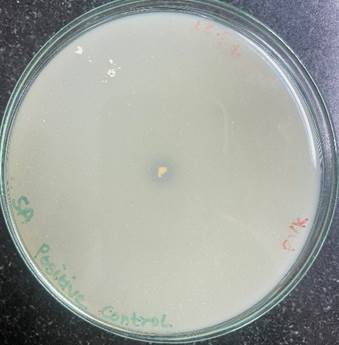
|
Figure 2 Positive
Control S. Aureus. |
The phosphate solubilization index (PSI) of the isolated bacterium and positive control bacterium S. aureus was found to be 3.33 and 1.45 respectively
3.2. Quantitative estimation of phosphate solubilization in pikovskaya’s broth medium
The isolated bacterial strain's capacity to solubilize phosphate was tested in broth culture. The results demonstrated that the amount of soluble P in the supernatants of the bacterial strain was 667.0 µg/ml after five days of incubation. The amount of phosphate solubilized by the positive control strain S. aureus was found to 131.0 µg/ml be. It was observed that the amount of soluble phosphate increased as the final pH of the broth media decreased from 7.0 to 4.30.
Figure 3

|
Figure 3 Blue Colour Solution Indicates Positive Phosphate Solubilization an Uninoculated
PVK Medium Was Used as a Control. |
3.3. Qualitative analysis of phosphate solubilization using bromocresol purple indicator dye
The isolated phosphate solubilizing bacterial strain was tested for an additional phosphate solubilization test using a bromocresol indicator in pikovskaya’s agar medium. A yellow zone of solubilization around the inoculated bacterium indicated positive phosphate solubilization.
Figure 4
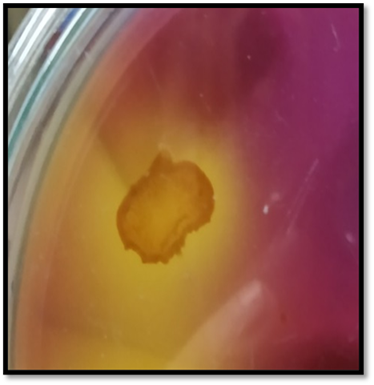
|
Figure 4 The Yellow Zone Around the Colony Represents Phosphate
Solubilization. |
3.4. Identification of phosphate solubilizing bacteria
Using MALDI-TOF-MS, the isolated phosphate solubilizing bacterium was identified at Himedia Laboratories in Mumbai. This sophisticated technique made it possible to identify the bacterial isolate and was regarded as a quick and precise way to identify bacteria. Klebsiella pneumoniae was identified as the bacterial isolate that displayed a score value of 9.662. With a score value higher than 9, a high level of precision is indicated.
Table 1
|
Table 1 MALDI-TOF Sequencing Score Values |
|
|
Bacterial samples for MALDI-TOF-MS |
Score |
|
Klebsiella pneumoniae |
9.662 |
|
Reference Staphylicoccus
aureus ATCC 6538 |
9.707 |
Figure 5
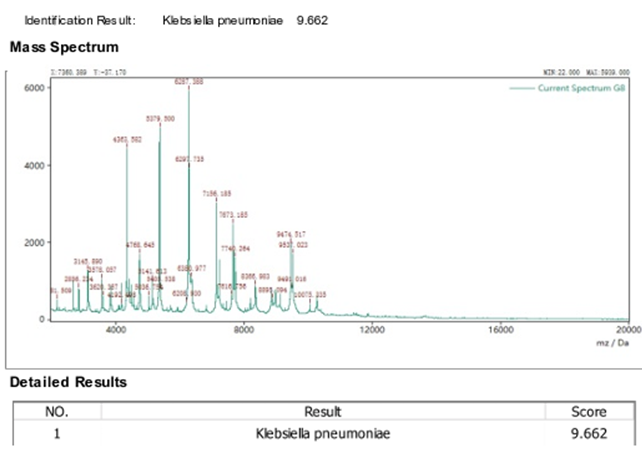
|
Figure 5 MALDI-TOF Sequencing |
3.5. Detection of organic acids by gas chromatography-mass spectrometry (GC-MS)
Organic acid production by the isolated phosphate solubilizing bacterium was investigated in PVK broth culture. GS-MS analysis of the culture filtrates revealed the presence of several organic acids that confer the capacity to solubilize insoluble TCP to the phosphate-solubilizing bacterial strain. The organic acids in the culture supernatant were found to be carbamic acid, dodecanoic acid, tetra decanoic acid, and trifluoroacetic acid.
Figure 6
|
Figure 6 GC-MS Chromatogram of Organic Acid Analysis |
Figure 7
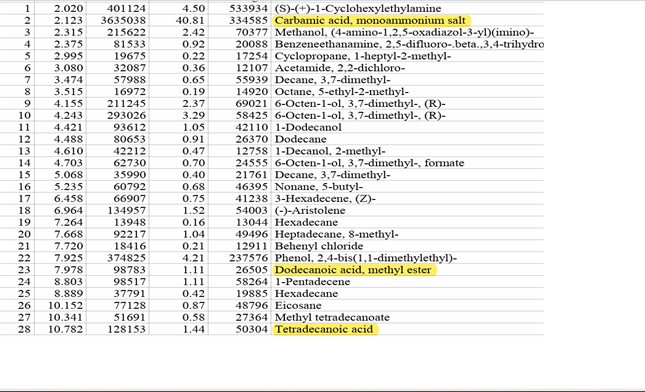
|
Figure 7 GC-MS Report of Organic Acid Analysis |
Figure 8
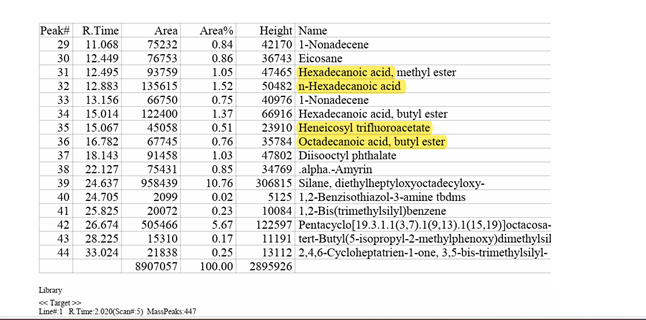
|
Figure 8 GC-MS Report of Organic Acid Analysis |
4. Discussion
Plant nutrient models have long demonstrated that slow
diffusion of inorganic phosphate (Pi) is a major barrier to P acquisition by
plants. Soil P is characterized by its restricted mobility when compared to
other important nutrients, such as nitrogen or potassium Janati et al. (2022). In the present study, Pikovskaya's media, which utilizes tricalcium
phosphate as the only source of phosphorus, was used for the initial screening
of the phosphate-solubilizing bacterial strain. Selecting an effective phosphate-solubilizing
bacterium is essential because it practically raises P in the rhizosphere of
plants. Based on its zone of solubilization, an effective
phosphate-solubilizing bacterial strain was isolated from a waste-dumping
ground soil sample in Mumbai. Phosphate solubilization based on its zone of
solubilization in pikovskaya’s medium has been previously reported by De
Campos et al. (2016). The zone of solubilization for the isolated phosphate solubilizing
bacterium and a positive control bacterium S. aureus was measured and
was found to be 3.33 and 1.45 respectively. When compared to the zone obtained
for the positive control S. aureus, it was found that the zone of
phosphate solubilization was significantly higher. The zone of solubilization
obtained was greater than 5mm on Pikovskaya’s agar plates. Similar findings
were reported in previous studies on the isolation of phosphate-solubilizing
bacteria Haouas et al. (2021).
Another qualitative assay to confirm phosphate solubilization was done using bromocresol purple indicator dye in pikovskaya’s agar medium. The plate was observed to produce a yellow zone around the bacterial colony Umeh & Sapele (2015). The appearance of a yellow zone of coloration around the bacterial colony is indicative of a decrease in pH due to the production of acids by the bacterium. Similar findings have been reported by Zheng et al. (2018).
Quantitative estimation of phosphate solubilization was carried out using the isolated phosphate solubilizing bacterial strain on a PVK liquid medium for 10 days. The phosphate solubilizing ability of the strain was increased from 0.0 mg/ml to 667.0 mg/ml as the pH decreased from 7.0 to 4.30. Similar findings were reported by Cao et al. (2018), Henri et al. (2008). Acidification of the media was shown to relate to phosphate solubilization. A primary factor linked to a drop in pH is the release of organic acids by bacteria that solubilize phosphate, which releases phosphate into the media by chelating cations attached to phosphate molecules Bolan et al. (1994), Mardad et al. (2013), Otani et al. (1996), Wei et al. (2018). Low molecular weight organic acids involving pqq genes are produced when phosphate solubilizing bacteria oxidize glucose. Some of these acids typically include gluconic acid, succinic acid, acetic acid, and formic acid. Ben Farhat et al. (2009). The isolated phosphate solubilizing bacterial strain demonstrated the production of organic acids such as carbamic acid, dodecanoic acid, tetra decanoic acid, and trifluoroacetic acid by GC-MS analytical technique. The findings of this study are like a previously reported study on organic acid production such as oxaloacetic acid, succinic acid, acetic acid, isovaleric acid, and caproic acid by phosphate solubilizing bacteria reported by Vazquez et al. (2000). The simultaneous production of different organic acids by the phosphate-solubilizing bacteria isolated in this study may enhance their potential for solubilizing insoluble phosphate.
This bacterial strain was identified as Klebsiella pneumoniae by MALDI-TOF-MS with a score value of 9.66. The score value above 9.5 indicated credible and accurate identification.
5. Conclusion
The main objective of this study was to isolate and identify phosphate solubilizing bacterium from the waste dumping ground in Mumbai. Phosphorus molecules become unavailable for plant uptake due to inorganic P fixation from insoluble complexes. Utilizing biofertilizers, like microbes, can aid with this by sustainably promoting plant development. Based on the above results, an efficient phosphate-solubilizing bacterium Klebsiella pneumoniae was isolated and evaluated for its phosphate-solubilizing potential. The organic acid analysis using GC-MS confirmed the presence of organic acids responsible for phosphate solubilization. To fully utilize these bacteria, further research on microbial-mineral interactions and genetic pathways is required. New innovative studies should concentrate on the ways that microbial biotechnology may be applied in agriculture to find new phosphate-solubilizing bacteria that can be used in a consortium to have competent microbial inoculants in systems for producing sustainable cultures under different circumstances.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors are likely to thank the Department of Life Science at Ramnarain Ruia Autonomous College for providing us with the laboratory Equipment and chemicals.
REFERENCES
Ben Farhat, M., Farhat, A., Bejar, W., Kammoun, R., Bouchaala, K., Fourati, A., Antoun, H., Bejar, S., & Chouayekh, H. (2009). Characterization of the Mineral Phosphate Solubilizing Activity of Serratia Marcescens CTM 50650 Isolated From the Phosphate Mine of Gafsa. Archives of Microbiology, 191(11), 815–824. https://doi.org/10.1007/s00203-009-0513-8
Bolan, N. S., Naidu, R., Mahimairaja, S., & Baskaran, S. (1994). Influence of Low-Molecular-Weight Organic Acids on the Solubilization of Phosphates. Biology and Fertility of Soils, 18(4), 311–319. https://doi.org/10.1007/BF00570634
Cao, Y., Fu, D., Liu, T., Guo, G., & Hu, Z. (2018). Phosphorus Solubilizing and Releasing Bacteria Screening from the Rhizosphere in a Natural Wetland. Water (Switzerland), 10(2), 1–15. https://doi.org/10.3390/w10020195
Chauhan, A., Guleria, S., Balgir, P. P., Walia, A., Mahajan, R., Mehta, P., & Shirkot, C. K. (2017). Tricalcium Phosphate Solubilization and Nitrogen Fixation by Newly Isolated Aneurinibacillus Aneurinilyticus CKMV1 from Rhizosphere of Valeriana Jatamansi and Its Growth Promotional Effect. Brazilian Journal of Microbiology, 48(2), 294–304. https://doi.org/10.1016/j.bjm.2016.12.001
De Campos, M., Antonangelo, J. A., & Alleoni, L. R. F. (2016). Phosphorus Sorption Index in Humid Tropical Soils. Soil and Tillage Research, 156, 110–118. https://doi.org/10.1016/j.still.2015.09.020
Haouas, A., el Modafar, C., Douira, A., Ibnsouda-Koraichi, S., Filali-Maltouf, A., Moukhli, A., & Amir, S. (2021). Alcaligenes Aquatilis GTE53: Phosphate Solubilising and Bioremediation Bacterium Isolated from New Biotope “Phosphate Sludge Enriched-Compost.” Saudi Journal of Biological Sciences, 28(1), 371–379. https://doi.org/10.1016/j.sjbs.2020.10.015
Henri, F., Laurette, N. G. O. N., Annette, D., & John, Q. (2008). Solubilization of Inorganic Phosphates and Plant Growth Promotion by Strains of Pseudomonas Fluorescens Isolated from Acidic Soils of Cameroon. African Journal of Microbiology Research, 2(7), 171–178. https://doi.org/10.1186/1471-2164-9-316
Janati, W., Mikou, K., El Ghadraoui, L., & Errachidi, F. (2022). Isolation and Characterization of Phosphate Solubilizing Bacteria Naturally Colonizing Legumes Rhizosphere in Morocco. Frontiers in Microbiology, 13(September). https://doi.org/10.3389/fmicb.2022.958300
Kadiri, D. D., Gorle, N., Varada, K., Peetala, R., & Peela, S. (2013). Isolation, Screening and Identification of Phosphate Solubilizing Bacteria From Different Regions of, 4(4), 518–526.
Karpagam, T., & Nagalakshmi, P. K. (2014). Isolation and Characterization of Phosphate Solubilizing Microbes from Agricultural Soil. Int.J.Curr.Microbiol. App.Sci, 3(3), 601–614.
Kaur, R., Kaur, R., Sharma, A., Kumar, V., Sharma, M., Bhardwaj, R., & Thukral, A. K. (2018). Microbial Production of Dicarboxylic Acids from Edible Plants and Milk Using GC-MS. Journal of Analytical Science and Technology, 9(1). https://doi.org/10.1186/s40543-018-0154-0
Kumar, A., Bhargava, P., & Rai, L. C. (2010). Isolation and Molecular Characterization of Phosphate Solubilizing Enterobacter and Exiguobacterium Species from Paddy Fields of Eastern Uttar Pradesh, India. African Journal of Microbiology Research, 4(9), 820–829.
Mahadevamurthy, M., Channappa, T., Sidappa, M., Raghupathi, M., & Nagaraj, A. (2016). Isolation of Phosphate Solubilizing Fungi from Rhizosphere Soil and Its Effect on Seed Growth Parameters of Different Crop Plants. Journal of Applied Biology and Biotechnology, 4(06), 022–026. https://doi.org/10.7324/jabb.2016.40604
Mardad, I., Serrano, A., & Soukri, A. (2013). Solubilization of Inorganic Phosphate and Production of Organic Acids by Bacteria Isolated from a Moroccan Mineral Phosphate Deposit. African Journal of Microbiology Research, 7(8), 626–635. https://doi.org/10.5897/AJMR12.1431
Noorjahan, A., Aiyamperumal, B., & Anantharaman, P. (2019). Isolation and Charecterisation of Seaweed Endophytic Fungi as an Efficient Phosphate Solubiizers. Biosciences, Biotechnology Research Asia, 16(1), 33–39. https://doi.org/10.13005/bbra/2718
Otani, T., Ae, N., & Tanaka, H. (1996). Phosphorus (P) Uptake Mechanisms of Crops Grown in Soils with Low P Status: II. Significance of Organic Acids in Root Exudates of Pigeonpea. Soil Science and Plant Nutrition, 42(3), 553–560. https://doi.org/10.1080/00380768.1996.10416324
Pande, A., Pandey, P., & Kaushik, S. (2017). Co-Inoculation of and Enhances Plant Growth of Maize () Under Green House and Field Condition. Korean Journal of Agricultural Science, 44(2), 196–210. https://doi.org/10.7744/kjoas.20170019
Pande, A., Pandey, P., Mehra, S., Singh, M., & Kaushik, S. (2017). Phenotypic and Genotypic Characterization of Phosphate Solubilizing Bacteria and Their Efficiency on the Growth of Maize. Journal of Genetic Engineering and Biotechnology, 15(2), 379–391. https://doi.org/10.1016/j.jgeb.2017.06.005
Rfaki, A., Nassiri, L., & Ibijbijen, J. (2015). Isolation and Characterization of Phosphate Solubilizing Bacteria from the Rhizosphere of Faba Bean (Vicia faba L.) in Meknes Region, Morocco. British Microbiology Research Journal, 6(5), 247–254. https://doi.org/10.9734/bmrj/2015/14379
Son, H. J., Park, G. T., Cha, M. S., & Heo, M. S. (2006). Solubilization of Insoluble Inorganic Phosphates by a Novel Salt- and pH-Tolerant Pantoea Agglomerans R-42 Isolated from Soybean Rhizosphere. Bioresource Technology, 97(2), 204–210. https://doi.org/10.1016/j.biortech.2005.02.021
Susilowati, L. E., Kusumo, B. H., & Arifin, Z. (2019). Screening of the Drought Tolerant Phosphate Solubilizing Bacteria in Dissolving P-Inorganic. Journal of Physics: Conference Series, 1402(5). https://doi.org/10.1088/1742-6596/1402/5/055082
Umeh, S. I., & Sapele, A. (2015). Effect of Phosphate Solubilizing Bacteria on. Nigerian Journal of Microbiology, 29(August), 3159–3166.
Vazquez, P., Holguin, G., Puente, M. E., Lopez-Cortes, A., & Bashan, Y. (2000). Phosphate-Solubilizing Microorganisms Associated with the Rhizosphere of Mangroves in a Semiarid Coastal Lagoon. Biology and Fertility of Soils, 30(5–6), 460–468. https://doi.org/10.1007/s003740050024
Wang, Z., Xu, G., Ma, P., Lin, Y., Yang, X., & Cao, C. (2017). Isolation and Characterization of a Phosphorus-Solubilizing Bacterium from Rhizosphere Soils and Its Colonization of Chinese Cabbage (Brassica Campestris ssp. Chinensis). Frontiers in Microbiology, 8(JUL), 1–12. https://doi.org/10.3389/fmicb.2017.01270
Wei, Y., Zhao, Y., Shi, M., Cao, Z., Lu, Q., Yang, T., Fan, Y., & Wei, Z. (2018). Effect of Organic Acids Production and Bacterial Community on the Possible Mechanism of Phosphorus Solubilization During Composting with Enriched Phosphate-Solubilizing Bacteria Inoculation. Bioresource Technology, 247, 190–199. https://doi.org/10.1016/j.biortech.2017.09.092
Zheng, B. X., Ibrahim, M., Zhang, D. P., Bi, Q. F., Li, H. Z., Zhou, G. W., Ding, K., Peñuelas, J., Zhu, Y. G., & Yang, X. R. (2018). Identification and Characterization of Inorganic-Phosphate-Solubilizing Bacteria from Agricultural Fields With a Rapid Isolation Method. AMB Express, 8(1). https://doi.org/10.1186/s13568-018-0575-6
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.