
HISTOCHEMICAL LOCALIZATION OF ACID AND ALKALINE PHOSPHATASES IN Moniezia expansa (Rudolphi, 1805)
1 Assistant
Professor, Department of Zoology, M.D.D.M. College, B.R.A. Bihar University,
Muzaffarpur, India
|
|
ABSTRACT |
||
|
Cestodes or true tapeworms are important group of the Platyhelminthes, endoparasitic in the alimentary tract and associated ducts of various vertebrates and therefore, of great interest to the medical and veterinary profession. Moniezia expansa, the tapeworm under study is the common parasite of oldest domesticated animal sheep. It inhabits the small intestine especially the lower portion and absorb the nutrition of host from the intestine and deprive it of many important nutrients and thus deteriorates the quality of meat, wool, skin of host which leads to ecomical losses as well. Phosphatases are enzymes hydrolysing esters of phosphoric acid and play an important role in many metabolic processes. The histochemical localization of acid and alkaline phosphatase has been studied in Moniezia expansa as an effort to study some of its biological aspects which would help in its control and chemotherapy. Acid phosphatase was found to be localized in tegument, subtegumental cells, parenchymal muscles, interproglottidal gland tegument, Mehlis glands, testes, uterine wall and eggs and was not observed in parenchyma, interproglottidal gland cells, excretory vessels, ovaries and vitellaria. Alkaline phosphatase activity was observed in tegument, subtegumental cells, parenchymal muscles, parenchyma, interproglottidal glands, excretory vessels, membranes enclosing ovaries, vitellaria, Mehlis gland, testes, uterine wall and eggs. The presence of acid phosphatase in testes, mehlis' glands, uterine wall and eggs as well as alkaline phosphatase in eggs in Moniezia expansa has been reported for the first time. Both the acid
and alkanine phosphatases showed the variations in
their activity in the tegument along the strobila with highest in mature
region. This gives the idea of high metabolic activity in the mature region
of the cestode. These phosphatases play important role in metabolism of the
parasite and seems to be involved in transportation, digestion, distribution
of nutrients and metabolites and absorption of metabolic end products. |
|||
|
Received 03 November 2023 Accepted 04 December
2023 Published 31 December 2023 Corresponding Author Dr.
Archana Gupta, archanaguptajrf@gmail.com DOI 10.29121/granthaalayah.v11.i12.2023.5398 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Phosphatases, Alkaline phosphatases, Acid
Phosphatases, Moniezia Expansa,
Cestode, Histochemistry, Enzyme |
|||
1. INTRODUCTION
Cestodes or true tapeworms are important group of the Platyhelminthes, endoparasitic in the alimentary tract and associated ducts of various vertebrates and therefore, of great interest to the medical and veterinary profession.
Moniezia expansa, the tapeworm under study is the common parasite of oldest domesticated animal sheep. It inhabits the small intestine especially the lower portion and absorb the nutrition of host from the intestine and deprive it of many important nutrients and thus deteriorates the quality of meat, wool, skin of host which leads to ecomical losses as well.
Phosphatases are enzymes hydrolysing esters of phosphoric acid and are widely distributed. They play an important role in many metabolic processes especially carbohydrate metabolism and in transphosphorylation transfer mechanisms of a number of helminth parasites. Phosphatases active in acidic pH are acid phosphatases and those in alkaline pH are alkaline phosphatases.
Histochemical distribution of phosphatases has been
performed in many cyclophyllidean cestodes. Rogers (1947), Sircar
and Sinha (1978), Yamao (1952), Kilejian et al. (1961), Erasmus
(1957ab), Waitz (1963), Bogitsh (1963),
Waitz and Schardein (1964), Lee
and Tatchell (1964), Howells and Erasmus (1969), Mayberry
and Tibbitts (1972), Moczon (1974), Roy
(1979), Khera
and Arora (1984), Farooq and Farooqi (1984), Upender and Bhargavi (1985), Varma et al. (1985), Suman et al (2021).
The presence of acid and alkaline phosphatases in cestode Moniezia expansa has been studied earlier by many workers (Rogers (1947), Yamao (1952), Erasmus (1957b), Howells and Erasmus (1969), Khera and Arora (1984) and Varma et al. (1985) but most of these studies are basically limited to tegument and interproglottidal glands.
Therefore, in the present work, an attempt has been made to study the histochemical localization of acid and alkaline phosphatases in various tissues and organs of scolex, immature, mature and gravid regions of Moniezia expansa.
2. MATERIALS AND METHODS
The parasites were collected from the intestine of sheep obtained from local abbatoir, washed in several times in normal saline till free of debris.
For histochemical localization of acid and alkaline phosphatases in Moniezia expansa, scolex, immature, mature and gravid regions of the tapeworm were fixed in absolute acetone for 24 hours at 4oC, blocks of 3 to 5 proglottids were embedded in paraffin wax. Longitudinal and transverse sections were cut at 6-8 µm.
For cold formalin fixed frozen sections, the fresh tissue sections at 10 µm thickness were cut using the cryocut microtome (Leica CM 1800) and were mounted on a clean slide. These were then fixed in 4% formalin at 4oC for 12 to 24 hrs.
For in toto preparations, immature, mature and gravid proglottids were fixed in 4% formalin for 12-24 hrs, washed well to remove the fixative, incubated in substrate media, dehydrated, cleared and mounted.
The histochemical localization of acid phosphatase was done by lead phosphate method by Gomori (1950) as in Pearse (1972).
The histochemical localization of alkaline phosphatase was demonstrated by Calcium cobalt method by Gomori as in Pearse, 1972.
The control sections were immersed in incubating mixture lacking the substrate and also some control sections were treated with boiling water before incubating in the incubating mixture.
The presence of acid phosphatase in the sections was indicated by brownish black colour. The control sections showed no such colour.
Sites of sections possessing alkaline phosphatase activity were stained brownish black in colour and control sections showed negative reaction.
3. RESULTS
Acid phosphatase was found to be present in tegument, subtegumental cells, parenchymal muscles, interproglottidal gland tegument, Mehlis' gland, testis, uterus wall and eggs in Moniezia expansa. It was not observed in parenchyma, interproglottidal gland cells, excretory vessels, membranes enclosing lobules of ovaries and vitellaria. Tegument showed the variation in enzyme activity along the strobila. In mature proglottid it showed highest activity whereas in scolex and immature proglottids moderate and in gravid proglottids it showed very low activity.
The enzyme showed no activity in the control sections.
Alkaline phosphatase was present in tegument, subtegumental cells, parenchymal muscles, parenchyma, interproglottidal gland tegument, interproglottidal gland cells, excretory vessels, membranes enclosing ovaries, vitellaria, Mehlis' glands, testis, uterine wall and eggs in Moniezia expansa. Alkaline phosphatases showed non-uniform activity in the tegument along the strobila of the parasite. It was found to be highest in mature proglottids as compared to other proglottids. The enzyme was found to be absent in the control sections.
The results are summarized in following table:
Table 1
|
Table 1 General Distribution and
Intensity of Acid and Alkaline Phosphatases Reaction in Various Structures of
Moniezia Expansa |
||||
Structures
|
Acid phosphatase |
Alkaline Phosphtase |
||
|
Test |
Control |
Test |
Control |
|
|
Tegument (Scolex) (Immature
proglottid) (Mature proglottid) (Gravid proglottid) |
++ ++ +++ + |
– – – – |
++ ++ +++ + |
– – – – |
|
Subtegumental cells |
++ |
– |
++ |
– |
|
Parenchymal muscles |
+++ |
– |
+++ |
– |
|
Parenchyma |
– |
– |
+ |
– |
|
Interproglottidal gland tegument |
+++ |
– |
+++ |
– |
|
Interproglottidal gland cells |
– |
– |
++ |
– |
|
Excretory vessels |
– |
– |
++ |
– |
|
Membrane enclosing lobules of ovaries |
– |
– |
++ |
– |
|
Vitellaria |
– |
– |
++ |
– |
|
Mehlis gland |
++ |
– |
+++ |
– |
|
Testis |
++ |
– |
++ |
– |
|
Uterus wall |
+ |
– |
+ |
– |
|
Egg |
++ |
– |
++ |
– |
|
(+++) Intense
reaction, (++) Moderate reaction, (+) Present, (–) Absent. |
||||
Figure 1

|
Figure 1 Control Sections of Mature Proglottids of Moniezia Expansa Showing Absence of Alkaline Phosphatase Activity. |
Figure 2

|
Figure 2 T.S of Mature Proglottid of Moniezia Expansa Showing Alkaline Phosphatase Activity Especially in the Tegument (1), Muscles (2) And Membrane Enclosing Ovaries (3). |
Figure
3

|
Figure 3 L.S of Gravid Proglottid of Moniezia Expansa Showing Acid
Phosphatase Activity. |
Figure 4
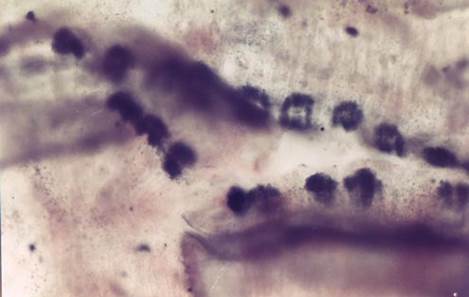
|
Figure 4 Whole Mount of Mature Proglottid
of Moniezia Expansa
Showing Acid Phosphatase Activity (Black Colour) in the Interproglottidal
Gland Tegument. |
Figure 5
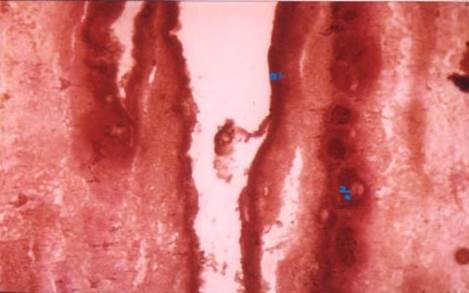
|
Figure 5 T.S of Mature Proglottids of Moniezia Expansa Showing Acid
Phosphatase Activity in The Tegument (1) and Interproglottidal
Gland Tegument (2) |
4. DISCUSSION
In the present study, both acid and alkaline phosphatases have been observed in the tegument of Moniezia expansa. These are in accordance to that of Erasmus (1957b), Howells and Erasmus (1969), Khera and Arora (1984), Varma et al. (1985) in Moniezia expansa and Erasmus (1957b), Waitz (1963), Bogitsh (1963), Waitz and Schardein (1964), Roy (1979), Farooq and Farooqi (1984), Varma et al. (1985), Upender and Bhargavi (1985) and Suman et. al. (2021).
The presence of alkaline phosphatase in tegument of Moniezia expansa is similar to Rogers (1947) in Moniezia expansa, Kilejian et al. (1961) in Echinococcus granulosus, Mayberry and Tibbitts (1972) in Hymenolepis diminuta and Sircar and Sinha (1978) in Raillietina echinobothrida.
Non-uniform activity of the phosphatases has been observed along the different (scolex, immature, mature and gravid) regions of strobila with mature region having highest activity in M. expansa. This was in conformity with Rogers (1947) for alkaline phosphatase and Erasmus (1957b), Howells and Erasmus (1969), Khera and Arora (1984) and Varma et al. (1985) for both phosphatases in Moniezia expansa. This has also been observed in other cestodes by Erasmus (1957a), Mayberry and Tibbitts (1972) and Roy (1979). This non-uniform distribution reflects the metabolic differences between proglottids at different stages.
The activities of phosphatases result in the release of phosphate ions, which may be utilized at cuticular (tegumental) surface for phosphorylated passage of substance through the cuticle or tegument Erasmus (1957). It has been well established that tegument is metabolically active and can take up, as well as digest nutrient through active transport Smyth (1969); Dike and Read (1971), which is accomplished by phosphohydrolases Roy (1979).
The observation of both acid and alkaline phosphatases in the subcuticular or subtegumental cells in Moniezia expansa by author is in accordance with that of Waitz (1963), Waitz and Schardein (1964), Roy (1979) and Suman et.al (2021). The presence of only alkaline phosphatase was reported by Erasmus (1957ab), Kilejian et al. (1961), Bogitsh (1963), Howells and Erasmus (1969), Sircar and Sinha (1978) in the subcuticular cells and this was similar to the present observation. Phosphatases in subtegumental cells may be associated with the secretory functions as these play an important role in formation of the syncytial protoplasmic layer of the tegument.
Rogers (1947), Sircar and Sinha (1978), Yamao (1952), Kilejian et al. (1961), Erasmus (1957ab), Waitz (1963), Bogitsh (1963), Waitz and Schardein (1964), Lee and Tatchell (1964), Howells and Erasmus (1969), Mayberry and Tibbitts (1972), Moczon (1974), Roy (1979), Khera and Arora (1984), Farooq and Farooqi (1984), Upender and Bhargavi (1985), Varma et al. (1985), Suman et al (2021)
Both acid and alkaline phosphatases activities were observed by author in the parenchymal muscles in Moniezia expansa. This was similar to Roy (1979), Farooq and Farooqi (1984), Upender and Bhargavi (1985) and Suman et.al (2021) who reported both acid and alkaline phosphates in parenchymal tissues. The presence of only alkaline phosphatases was reported by Erasmus (1957ab). Keljian et al. (1961) Bogitsh (1963), Waitz (1963), Waitz and Schardein (1964), Howells and Erasmus (1969) and Sircar and Sinha (1978). The present observations are not in accordance with Erasmus (1957), Bogitsh (1963), Waitz (1963), Waitz and Schardein (1964), Howells and Erasmus (1969) who failed to observe acid phosphatase activity in muscles in studied cestode models.
Parenchyma in the present study was found to have very low activity for alkaline phosphatase and acid phosphatase showed no activity. This has been in accordance to Waitz and Schardein (1964). Different workers reported the presence of acid phosphatases in parenchyma viz. Roy (1979) and Farooq and Farooqi (1984).
The presence of alkaline phosphatases has been observed in excretory canals of Moniezia expansa. This is in accordance to Erasmus (1957), Bogitsh (1963), Lee and Tatchell (1964), Mayberry and Tibbitts (1972), Sircar and Sinha (1978), Roy (1979) and Farooq and Farooqi (1984) in different cestodes. But the absence of acid phosphatase in excretory canal in M.expansa in present study by author was different from that of Roy (1979) and Farooq and Farooqi (1984) who reported acid phosphatase in the excretory canals of Raillietina (R.) johri and Avitellina lahorea respectively. The presence of phosphatases along excretory canal suggests its role in absorption of metabolic end products and distribution of nutrients to different parts of Moniezia expansa Howells (1969).
Acid and alkaline phosphatases showed intense reaction in the interproglottidal glands tegument. It was observed that acid phosphatase has been absent and alkaline phosphatase being present in the gland cells of interproglottidal gland in M. expansa. These findings were similar to the findings of other authors.
Observation of phosphatases in reproductive organs are significant and in accordance to those reported by various workers in different cestodes Erasmus (1957ab), Moczon (1974), Sircar and Sinha (1978), Roy (1979), Farooq and Farooqi (1984), Upender and Bhargavi (1985). Acid phosphatases were observed for the first time in testes, Mehlis' glands, uterine wall and eggs in Moniezia expansa. Alkaline phosphatase activity has been observed in membranes enclosing lobules of ovaries, testes, vitellaria, uterine wall and eggs. Both acid and alkaline phosphatases in eggs are reported for the first time. However, the present observations are different from those of Kilejian et al. (1961), Bogitsh (1963), Waitz (1963), Waitz and Schardein (1964), who did not observe the acid or alkaline phosphatases in the reproductive organs of respective cestodes under their studies.
Phosphatases in reproductive organs might be involved in transport of metabolites in addition to bring important components of cells enforced in rapid synthesis. Moczon (1974) suggested that the presence of acid phosphatase in spermatozoa of Hymenolepis diminuta might facilitate the formation of lysosome. This may be the same for Moniezia expansa also. In mature spermatozoa this enzyme may help in the breakdown of non-specific phosphate ester in order to maintain a phosphate pool for the production of high energy-yielding compounds Anderson et al. (1968).
Thus, the presence of phosphatases in tegument, reproductive organs, interproglottidal glands and excretory canals seem to play an important role in metabolism of cestode Moniezia expansa.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
Financial support by CSIR is acknowledged.
REFERENCES
Anderson, W.A., Personne, P. and Andre, J. (1968). Chemical Compartmentalization in Helix Spermatozoa. J. Microsc. 7, 367-390.
Dike, S.C. and Read, C.P. (1971). Relations of Tegumentary Phosphohydrolase and Sugar Transport in Hymenolepis Diminuta. J. Parasitol. 57, 1251-1255. https://doi.org/10.2307/3277975
Erasmus, D.A. (1957b). Studies on Phosphatase Systems of Cestodes. II Studies on Cysticercus Tenuicollis and Moniezia Expansa (Adult), Parasitology 47, 81-91. https://doi.org/10.1017/S0031182000021788
Farooq, R. and Farooqi, H.U. (1984). Histochemical Localization of Phosphomonoesterases in Avitellina lahorea. Woodland, 1927 (Cestoda: Anoplocephalida). J. Helminthol. 58, 169-173. https://doi.org/10.1017/S0022149X00028716
Howells, R.E. (1969). Observation on the Nephridial System of the Cestoda Moniezia expansa (Rud., 1805). Parasitology, 59(2), 449-459. https://doi.org/10.1017/S0031182000082408
Howells, R.E. and Erasmus, D.A. (1969). Histochemical Observations on the Tegumental Epithelium and Interproglottid Glands of Moniezia Expansa (Rud., 1805) (Cestoda, Cyclophyllidea). Parasitology, 59, 505-518. https://doi.org/10.1017/S0031182000031073
Khera, S. and Arora, S. (1984). Histochemical Studies of Certain Enzymes in Interproglottidal Glands of Moniezia Expansa. Indian. J. Parasitol. 8(2), 281-283.
Kundu, S., Mandal, S., Mondal, C. and Lyndem, L. M. (2021). Alteration in the Tegumental Enzymes of Hymenolepis diminuta by Senna spp". Acta Scientific Microbiology 4 (111), 39-45. https://doi.org/10.31080/ASMI.2021.04.0949
Lee, D.L and Tatchell, R.J. (1964). Studies on the Tapeworm Anoplocephala Perfoliata (Goeze, 1782). Parasitology. 54, 467-479. https://doi.org/10.1017/S0031182000082512
Mayberry, L.F. and Tibbitts, F.D. (1972). Hymenolepis Diminuta (Order Cyclophyllidea): Histochemical Localization of Glycogen, Neutral Lipids and Alkaline Phosphatase in Developing Worms. Z. Parasitenk. 38, 66-76. https://doi.org/10.1007/BF00259486
Pearse, A.G.E. (1968). Histochemistry Theoretical and Applied. Vol. I. 3rd Edition, J. and A. Churchill Ltd., London.
Roy, T.K. (1979). Histochemical Studies on Raillietina (Raillietina) Johri (Cestoda: Davaineidae). I. Non-specific and Specific Phosphatases. J. Helminthol. 53, 45-49. https://doi.org/10.1017/S0022149X00005733
Smyth, J.D. (1969). The Physiology of Cestodes. Ist edition. Oliver and Boyd. Edinburgh.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.