
THE EFFECT OF CATECHINS AND NANOCHITOSAN ON REDUCING BACTERIAL COLONIES AND MATERIAL PERFORMANCE IN PACKAGING FILMS BASED ON PLA/PCL BLEND
Suryani Salim 1![]() , Teuku Rihayat 1
, Teuku Rihayat 1![]() , Fitria 2
, Fitria 2![]() , Aida Safitri 3
, Aida Safitri 3![]()
1 Department
of Chemical Engineering, Politeknik Negeri Lhokseumawe, Lhokseumawe, Aceh
24301, Indonesia
2 Department
of Dermato Venereology, Medical Faculty, Syiah Kuala
University, Aceh 23111, Indonesia
3 Department of Chemical Engineering, Faculty of Engineering,
Universitas Sumatera Utara, Kota Medan 20222, Indonesia
|
|
ABSTRACT |
||
|
The extensive
utilization of petrochemical polymer-based plastics has led to significant
environmental challenges. A viable solution involves the incorporation of
high-quality biomaterials as a substitute for traditional plastics. In
pursuit of this goal, Polylactic Acid (PLA) and Polycaprolactone (PCL)
biopolymers were combined with catechin and nano chitosan additives to
innovate food packaging materials. The process involved the utilization of a
screw extruder for mixing and melting. The sample formulation employed a
combination of PLA polymer (9.5 g) and PCL (0.5 g). The variations in
catechin (0%, 5%, 10%, 15%, 20%, 25%, and 30%), while nano chitosan was added
in concentrations (5%, 10%, 15%, 20%, 25%, and 30%). The highest tensile
strength recorded, at 45.10 MPa, was achieved by sample SA4, as indicated by
tensile strength testing, FTIR analysis, and colony reduction. FT-IR analysis
revealed the presence of functional groups, namely N-H, C-H, C=O, and C-O,
signifying successful interactions between the PLA/PCL matrix blend and the
additive components of nanochitosan and catechins.
Remarkably, sample SA4 exhibited a remarkable 96% reduction in S. aureus
bacterial colonies following 24 hours of storage. |
|||
|
Received 29 August 2023 Accepted 30 September 2023 Published 17 October 2023 Corresponding Author Suryani
Salim, suryani.salimpnl@gmail.com DOI 10.29121/granthaalayah.v11.i9.2023.5334 Funding: The author
expresses his gratitude and highest appreciation to Sincere appreciation to
the Ministry of Research, Technology and Higher Education of the Republic of
Indonesia and Lhokseumawe State Polytechnic which
has funded through grants number: 461/BAP/SPK/04/PPK.01.APTV/VI/PNL/2023. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: PLA, PCL, Antimicrobial Activity,
Catechin, Mechanical Properties |
|||
1. INTRODUCTION
Plastic has become an indispensable material in our daily lives, owing to its strength, lightweight nature, and affordability. It is widely employed in various forms, including packaging for beverages, food, and shopping bags Safitri et al. (2022). However, the escalating accumulation of non-renewable plastic waste poses significant environmental and human challenges. Moreover, the depletion of petroleum resources, a key component in plastic production, necessitates sustainable alternatives that are readily available in nature, eco-friendly, and cost-effective.
The environmental impact of petroleum-based plastic packaging underscores the urgency for the development of natural biopolymers. One such bioplastic with properties akin to synthetic plastics is Polylactic Acid (PLA). PLA is a biopolymer derived from renewable resources, such as starch through lactic acid fermentation and chemical polymerization. It offers strength, transparency, and waterproof characteristics but is hindered by rigidity and low permeability Rihayat et al. (2022), Rihayat et al. (2021). To address these limitations, researchers have explored the incorporation of additional polymers like PBAT, Polyhydroxyalkanoates (PHAs), and Cellulose-Based Materials into PLA composites. This additive approach aims to enhance PLA's properties and augment the packaging film's overall performance. Another polymer with the potential to improve PLA's attributes is Polycaprolactone (PCL), a synthetic polymer with excellent biodegradability. While both PLA and PCL share hydrophobicity and biodegradability, they differ in physical characteristics Ncube et al. (2020), Yu et al. (2021), Wu et al. (2022), An et al. (2023).
PLA is highly transparent but rigid with low permeability, while PCL lacks transparency but exhibits flexibility and strength De la Rosa-Ramírez et al. (2023). Combining these two polymers yields a composite that addresses the shortcomings of each component. The production of PLA/PCL blends results in packaging films that maintain their structural integrity as PCL concentration increases, alongside a reduction in crystallinity Bianchi et al. (2023), Luckachan et al. (2011).
Advanced food packaging technology plays a pivotal role in ensuring food safety by shielding products from contaminants and pathogenic microorganisms, thereby extending the shelf life of preserved foods. Nanochitosan was chosen as an antibacterial agent for active packaging due to its exceptional antimicrobial properties, biocompatibility, and biodegradability. Its nano-sized particles offer a large surface area for interaction with bacteria, enhancing its antibacterial efficacy. Moreover, its film-forming capability makes it an ideal candidate for incorporating into packaging materials, providing a physical barrier against microbial contamination Ganewatta et al. (2021) . Another additive like catechins, which is part of polyphenolic compounds found in various plants, also possess antibacterial properties Martinez Villadiego et al. (2021). Prior research efforts have explored the incorporation of catechin additives to enhance packaging film properties. In a recent, utilization zein-based active packaging films incorporating catechin exhibited notable reductions in water vapor permeability and swelling degree, while tensile strength and elongation at the break increased significantly Kumari et al. (2022). Another investigated the utilization of catechins in producing films based on starch conjugates with aldehydes, resulting in improved water vapor permeability, tensile strength, and elongation at break Iglesias-Montes et al. (2022). Furthermore, the previous report state that polylactic acid and catechins from tea to develop active packaging materials effectively inhibited Staphylococcus aureus and Escherichia coli for nearly 5 hours Edward et al. (2022). Therefore, this study aims to create packaging films based on PLA/PCL/Nanochitosan/Catechin polymer blends, focusing on their unique properties and antimicrobial properties.
2. MATERIALS AND METHODS
2.1. Materials
PLA (Polylactic Acid), PCL (Polycaprolactone), Catechins, Nanochitosan are purchased from PT Fugha Pratama Mandiri, Lhokseumawe, Aceh. Bacterial Cultures S.aureus supply by Faculty of pharmacy, Universitas Sumatera Utara, Medan, Indonesia.
2.2. Methods
2.2.1. Sample Preparation
The polymers, including PLA, PCL, nanochitosan, and Catechin, were quantified based on predetermined parameters as mention in Table 1. All materials were put inside the extruder. The blending process of these polymers was executed within a single-screw extruder at a temperature of 160 oC for 45 minutes. The polymer blend was subsequently poured into a mold conforming to ASTM 638 D Type I Standard, which had been lined with aluminum foil. Compression was then applied using a hot press at 180 0C for 20 minutes under atmospheric pressure. The resulting film was cooling down at room temperature, after which it underwent tensile strength mechanical property testing utilizing the Universal Tensile Machine (UTM) Exceed Model E43. The film were also characterized using FTIR and colony counter.
Table 1
|
Table 1 Sample Preparation |
|||
|
Sample Code |
PLA/PCL (g/g) |
Kitosan (g) |
Katekin (mL) |
|
SA1 |
9.5/0.5 |
5% |
25% |
|
SA2 |
9.5/0.5 |
10% |
20% |
|
SA3 |
9.5/0.5 |
15% |
15% |
|
SA4 |
9.5/0.5 |
20% |
10% |
|
SA5 |
9.5/0.5 |
25% |
5% |
|
SA6 |
9.5/0.5 |
30% |
0% |
|
SA7 |
9.5/0.5 |
- |
30% |
|
SA8 |
9.5/0.5 |
- |
- |
|
SA9 |
9.5 |
- |
- |
2.3. Characterization technique
2.3.1. Tensile Strength
The specimen secured in the grips of a universal testing machine (UTM). Gradually apply a tensile load to the specimen at a constant rate (typically specified in ASTM D-638) until the material fails or ruptures. During the test, record data such as load (force) and elongation (deformation) continuously. These data points will be used to generate a stress-strain curve.
2.3.2. FT-IR (Fourier Transform Infrared)
Fourier Transform Infrared (FT-IR) is used to identify the type of functional group bonding possessed
by materials including plastics. The purpose of FT-IR analysis on plastic film
samples is to see the wavelength and characteristic peaks in the sample. This
wavelength indicates the presence of certain functional groups in the sample,
because each functional group sample has a characteristic peak that is specific
to that functional group.
2.3.3. Colony reduction
Initially, the
S. aureus strain, which had been procured for experimental purposes, was
securely stored within an incubator under controlled conditions. To initiate
the experiment, one gram of the S. aureus culture was carefully mixed with a 10
mL solution of Potato
Dextrose Agar (PDA). This concoction was then incubated at a stable temperature
of 37°C for a duration of 24 hours to facilitate bacterial growth. Following the initial
incubation period, the bacterial culture was subsequently subjected to another
24-hour incubation cycle, this time at a slightly lower temperature of 33°C.
This step aimed to ensure the stability of the bacterial culture and its
readiness for subsequent testing. Following the second incubation period, the
bacterial samples were meticulously analyzed using a colony counter to
determine their population. Upon obtaining the initial count of bacterial
colonies, the culture medium was introduced to a solution containing
nanochitosan and catechin. This marked the introduction of the experimental
additives to assess their effects on the bacterial activity. Following this
crucial step, the bacterial culture underwent yet another 24-hour incubation
period. This final incubation phase was integral to ascertain any changes in
the number of bacterial colonies, specifically focusing on the bacterial
colonies that had been impacted or inhibited by the presence of nanochitosan
and catechin additives.
3. RESULTS AND DISCUSSIONS
3.1. Tensile Strength
The tensile strength analysis aims to determine the influence of PLA/PCL/nanochitosan/catechin variations on the tensile strength and percentage elongation of the resulting plastic films. One key finding of this study is that the addition of catechin and nanochitosan as reinforcements leads to a significant increase in the tensile strength of the produced nanocomposite plastic.
Figure 1
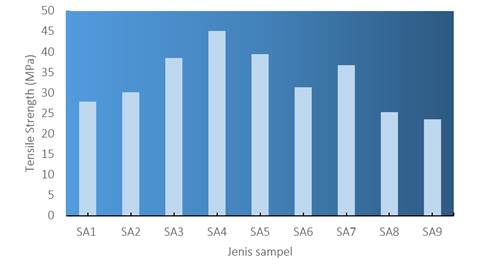
|
Figure 1 Tensile Strength of Film |
The addition of catechin as one of the natural antioxidant sources will act as a reinforcing agent, enhancing intermolecular interactions within the polymer matrix. This strengthening effect results in an increased load-bearing capacity and resistance to deformation under pressure Ranjbar-Mohammadi & Nouri (2022), Jiang et al. (2022). Its combination with nanochitosan, characterized by its amino and hydroxyl functional groups, exhibits excellent chemical compatibility with the polymer matrix. This compatibility facilitates strong interfacial bonding between chitosan and polymer chains, contributing to the overall tensile strength of the resulting films Hu et al. (2022). In Figure 1, the combination of catechin and nanochitosan also creates a synergistic effect further enhancing the tensile strength, as observed in the SA4 sample (45.1 MPa). Catechin's ability to neutralize free radicals and inhibit oxidation processes complements the strengthening properties of chitosan, resulting in a stronger polymer network.
Nanochitosan is chitosan that has been transformed into nanoparticles. This provides a larger surface area and excellent dispersion properties within the polymer matrix, such as PLA/PCL. Nanochitosan also possesses nanometer-sized particles, giving it a greater ability to reinforce the polymer matrix. The uniform distribution of nanochitosan within the matrix can effectively enhance tensile strength, elastic modulus, and impact resistance compared to regular chitosan
3.2. FTIR of
bioplastic
This functional
group analysis was conducted to identify the functional groups present in the
plastic film sample using Fourier Transform Infrared (FT-IR) spectroscopy. The
analysis is based on the characteristic peak wavelengths of a sample. These
peak wavelengths indicate the presence of specific functional groups in the
sample because each functional group has a specific characteristic peak. The
spectrum resulting from the FT-IR analysis can be seen in the image below.
Figure 2

|
Figure 2 FT-IR Spectrum of Sample SA4 |
Table 2
|
Table 2 FTIR Test Observation Data |
||
|
Frequency |
Absorption (cm-1) |
Functional Group |
|
3140,11 |
N – H Stretching |
|
|
3000 - 2850 |
2964,59 |
C – H Stretching |
|
2500 - 2000 |
2355,08 |
C = O Stretching |
|
1500 - 1250 |
1454,33 |
C – O Stretching |
From Figure 2, it is evident
from the best sample blend (SA4) in the conducted research that there is an
absorption at 3140.11 cm⁻¹, which confirms the presence of the N-H group.
This aligns with the literature, which states that the absorption band
appearing in the 3300-3500 cm⁻¹ range signifies the N-H group's presence,
indicating the existence of nanochitosan.
Additionally, in the 3000-2850 cm⁻¹ range, there is an absorption at
2964.59 cm⁻¹, indicating the presence of the C-H group. In the 2500-2000
cm⁻¹ range, an absorption at 2355.08 cm⁻¹ suggests the presence of
the C=O group, and in the 1500-1250 cm⁻¹ range, an absorption at 1454.33
cm⁻¹ indicates the presence of the C-O group, indicative of catechin groups.
These results demonstrate the functional groups that constitute the plastic
polymer. The results of the functional group identification in Table 2. indicate that
among all the functional groups detected, they correspond to the base materials
used, namely PLA, PCL, nanochitosan, and catechin,
without the formation of any new functional groups. Therefore, it can be
concluded that the plastic film manufacturing process proceeded flawlessly as
the constituent components were successfully identified Zafar et al. (2022), Tian et al. (2022).
3.3. Colony reduction
Nanochitosan has the scientific capability to reduce Staphylococcus aureus (S. aureus) colonies due to various factors, including its physicochemical properties and antimicrobial mechanisms. S. aureus is a pathogenic bacterium often responsible for human infections, including skin infections, respiratory tract infections, and food poisoning. Nanochitosan is a derivative of chitosan, a natural polysaccharide obtained from shrimp and crab exoskeletons Sadeghi et al. (2022), Gharehasanloo et al. (2023).
Figure 3

|
Figure 3 Colony Reduction of S.aureus in SA4 |
Chitosan possesses strong antimicrobial properties due to its positive charge. Nanochitosan, with its very small particle size, offers a large surface area, allowing it to interact with a greater number of S. aureus bacteria. The amine groups in chitosan's structure interact with the phosphate groups on the S. aureus bacterial cell walls, disrupting cell membrane integrity and permeability, inhibiting growth, and causing cell lysis. Furthermore, nanochitosan can penetrate bacterial cell membranes, leading to more extensive internal damage Swetha et al. (2023). Chitosan in nano-sized form, at a weight percentage of 20%, enables easier penetration of bacterial aggregates and biofilms that may form due to S. aureus. When nanochitosan successfully penetrates bacterial biofilms, they can directly interact with the protected bacterial cells within. This is significant because S. aureus often forms biofilms that reduce the effectiveness of conventional antibiotics. Good dispersion of nanochitosan in solution also allows for even distribution in the environment surrounding the bacteria, enhancing their ability to interact with a large number of S. aureus bacterial cells Wu et al. (2022), Moreno-Serna et al. (2023), Chia et al. (2023), Ertek et al. (2023), Zhu et al. (2022).
4. CONCLUSIONS AND RECOMMENDATIONS
The best results for mechanical properties, specifically tensile strength, were obtained from sample SA4 (9.5PLA/0.5PCL/20chitosan/10catechin). The addition of catechin and the distribution of nanochitosan enhance intermolecular interactions within the polymer matrix. This strengthening effect leads to an increased load-bearing capacity and resistance to deformation under pressure. FT-IR testing revealed the presence of functional groups N-H, C-H, C=O, C-O, indicating that the matrix blend of PLA/PCL successfully interacted with the additive components of nanochitosan and catechins. The S. aureus bacterial colonies in sample SA4 were capable of reducing bacterial colonies by 96% after being stored for 24 hours. The presence of nanochitosan and catechin enhanced cell permeability, which is associated with genomic DNA, causing cellular components of S. aureus to leak, ultimately leading to the death of bacterial colonies.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
An, L., Perkins, P., Yi, R., & Ren, T. (2023). Development of Polylactic Acid Based Antimicrobial Food Packaging Films with N-halamine Modified Microcrystalline Cellulose. International Journal of Biological Macromolecules, 242. https://doi.org/10.1016/j.ijbiomac.2023.124685.
Bianchi, M., Dorigato, A., Morreale, M., & Pegoretti, A. (2023). Evaluation of the Physical and Shape Memory Properties of Fully Biodegradable Poly (Lactic Acid) (PLA)/Poly (Butylene Adipate Terephthalate) (PBAT) Blends. Polymers, 15(4), 881. https://doi.org/10.3390/polym15040881.
Chia, Z. C., Chen, Y. L., Chuang, C. H., Hsieh, C. H., Chen, Y. J., Chen, K. H., Huang, T.-C., M.-C., Chen, & Huang, C. C. (2023). Polyphenol-Assisted Assembly of Au-Deposited Polylactic Acid Microneedles for SERS Sensing and Antibacterial Photodynamic Therapy. Chemical Communications, 59(42), 6339-6342. https://doi.org/10.1039/d3cc00733b.
De la Rosa-Ramírez, H., Aldas, M., Ferri, J. M., Pawlak, F., López-Martínez, J., & Samper, M. D. (2023). Control of Biodegradability Under Composting Conditions and Physical Performance of Poly (Lactic Acid) Based Materials Modified with Phenolic-Free Rosin Resin. Journal of Polymers and the Environment, 1-15. https://doi.org/10.1007/s10924-023-02956-1.
Edward, M. S. G., Louis, A. C. F., Srinivasan, H., & Venkatachalam, S. (2022). A Mechanochemical Approach for Synthesizing Almond Shell Nanoparticles and their Potential Application on the Enhancement of Polylactic Acid Film Properties. Iranian Polymer Journal, 31(12), 1523-1535. https://doi.org/10.1007/s13726-022-01099-9.
Ertek, D. A., Sanli, N. O., Menceloglu, Y. Z., & Seven, S. A. (2023). Environmentally Friendly, Antibacterial Materials from Recycled Keratin Incorporated Electrospun PLA Films with Tunable Properties. European Polymer Journal, 185. https://doi.org/10.1016/j.eurpolymj.2022.111804.
Ganewatta, M. S., Wang, Z., & Tang, C. (2021). Chemical Syntheses of Bioinspired and Biomimetic Polymers Toward Biobased Materials. Nature Reviews. Chemistry, 5(11), 753–772. https://doi.org/10.1038/s41570-021-00325-x.
Gharehasanloo, M., Anbia, M., & Yazdi, F. (2023). Preparation of Superhydrophobic, Green, and Eco-Friendly Modified Polylactic Acid Foams for Separation Oil from Water. International Journal of Biological Macromolecules, 240. https://doi.org/10.1016/j.ijbiomac.2023.124159.
Hu, H., Yong, H., Yao, X., Chen, D., Kan, J., & Liu, J. (2022). Effect of Starch Aldehyde-Catechin Conjugates on the Structural, Physical and Antioxidant Properties of Quaternary Ammonium Chitosan/Polyvinyl Alcohol Films. Food Hydrocolloids, 124. https://doi.org/10.1016/j.foodhyd.2021.107279.
Iglesias-Montes, M. L., Soccio, M., Siracusa, V., Gazzano, M., Lotti, N., Cyras, V. P., & Manfredi, L. B. (2022). Chitin Nanocomposite Based on Plasticized Poly (Lactic Acid) / Poly (3-hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials. Polymers, 14(15). https://doi.org/10.3390/polym14153177.
Jiang, L., Liu, F., Wang, F., Zhang, H., & Kang, M. (2022). Development and Characterization of Zein-Based Active Packaging Films Containing Catechin Loaded β-cyclodextrin Metal-Organic Frameworks. Food Packaging and Shelf Life, 31. https://doi.org/10.1016/j.fpsl.2022.100810.
Kumari, S. V. G., Pakshirajan, K., & Pugazhenthi, G. (2022). Recent Advances and Future Prospects of Cellulose, Starch, Chitosan, Polylactic Acid and Polyhydroxyalkanoates for Sustainable Food Packaging Applications. International Journal of Biological Macromolecules, 221, 163-182. https://doi.org/10.1016/j.ijbiomac.2022.08.203.
Luckachan, G. E., & Pillai, C. K. S. (2011). Biodegradable Polymers-A Review on Recent Trends and Emerging Perspectives. Journal of Polymers and the Environment, 19(3), 637-676. https://doi.org/10.1007/s10924-011-0317-1.
Martinez Villadiego, K., Arias Tapia, M. J., Useche, J., & Escobar Macías, D. (2021). Thermoplastic Starch (TPS)/Polylactic Acid (PLA) Blending Methodologies: A Review. Journal of Polymers and the Environment, 1-17. https://doi.org/10.1007/s10924-021-02207-1.
Moreno-Serna, V., Oyarzún, C., Ulloa-Flores, M. T., Rivas, L., Sepúlveda, F. A., Loyo, C., Toro, E.L., & Zapata, P. A. (2023). Venus Antiqua Clamshell-Derived Calcium Oxide Nanoparticles for the Preparation of PLA/d-Limonene/CaO Nanocomposites with Antimicrobial Properties. ACS Sustainable Chemistry & Engineering, 11(29), 10755-10766. https://doi.org/10.1021/acssuschemeng.3c01358.
Ncube, L. K., Ude, A. U., Ogunmuyiwa, E. N., Zulkifli, R., & Beas, I. N. (2020). Environmental Impact of Food Packaging Materials : A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials (Basel, Switzerland), 13(21), 4994. https://doi.org/10.3390/ma13214994.
Ranjbar-Mohammadi, M., & Nouri, M. (2022). Production and in Vitro Analysis of Catechin Incorporated Electrospun Gelatin/Poly (Lactic Acid) Microfibers for Wound Dressing Applications. Journal of Industrial Textiles, 51(5_suppl), 7529S-7544S. https://doi.org/10.1177/15280837211060883.
Rihayat, T., Aidy, N., Safitri, A., & Aida, A. (2022). Synthesis of Poly Lactic Acid (PLA)/Nanochitosan–Based for Bioscaffold Materials with the Addition of Zn-Curcumin. Materials Today : Proceedings, 63, S526-S531. https://doi.org/10.1016/j.matpr.2022.04.932.
Rihayat, T., Hadi, A. E., Aidy, N., Safitri, A., Siregar, J. P., Cionita, T., Irawan, A. P., et al. (2021). Biodegradation of Polylactic Acid-Based Bio Composites Reinforced with Chitosan and Essential Oils as Anti-Microbial Material for Food Packaging. Polymers, 13(22). http://dx.doi.org/10.3390/polym13224019.
Sadeghi, A., Razavi, S. M. A., & Shahrampour, D. (2022). Fabrication and Characterization of Biodegradable Active Films with Modified Morphology Based on Polycaprolactone-Polylactic Acid-Green Tea Extract. International Journal of Biological Macromolecules, 205, 341-356. https://doi.org/10.1016/j.ijbiomac.2022.02.070.
Safitri, A., Sinaga, P. S. D., Nasution, H., Harahap, H., Masyithah, Z., & Hasibuan, R. (2022). The Role of Various Plastisizers and Fillers Additions in Improving Tensile Strength of Starch-Based Bioplastics : A Mini Review. In IOP Conference Series : Earth and Environmental Science, 1115(1), http://dx.doi.org/10.1088/1755-1315/1115/1/012076.
Swetha, T. A., Ananthi, V., Bora, A., Sengottuvelan, N., Ponnuchamy, K., Muthusamy, G., & Arun, A. (2023). A Review on Biodegradable Polylactic Acid (Pla) Production from Fermentative Food Waste-its Applications and Degradation. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2023.123703.
Tian, J., Cao, Z., Qian, S., Xia, Y., Zhang, J., Kong, Y., Sheng, K., Zhang, Y., Wan, Y. & Takahashi, J. (2022). Improving Tensile Strength and Impact Toughness of Plasticized Poly (Lactic Acid) Biocomposites by Incorporating Nanofibrillated Cellulose. Nanotechnology Reviews, 11(1), 2469-2482. https://doi.org/10.1515/ntrev-2022-0142.
Wu, J., Liu, S., Wu, G., Zhang, M., Jing, Y., Li, J., Patowary, M.M.H., Chakma, R., Wang, C., Li, F., Jia, L., Zhang, Y., & Lu, D. (2022). Preparation and Properties of Polylactic Acid (PLA) Antibacterial Nanofiber Membrane with Ag@ TP Composite Antibacterial Agent. The Journal of the Textile Institute, 1-11. https://doi.org/10.1080/00405000.2022.2150951.
Wu, Y., Ma, Y., Gao, Y., Liu, Y., & Gao, C. (2022). Poly (Lactic Acid) - Based ph Responsive Membrane Combined with Chitosan and Alizarin for Food Packaging. International Journal of Biological Macromolecules, 214, 348–359. https://doi.org/10.1016/j.ijbiomac.2022.06.039.
Yu, F., Fei, X., He, Y., & Li, H. (2021). Poly (Lactic Acid) - Based Composite Film Reinforced with Acetylated Cellulose Nanocrystals and ZnO Nanoparticles for Active Food Packaging. International Journal of Biological Macromolecules, 186, 770–779. https://doi.org/10.1016/j.ijbiomac.2021.07.097.
Zafar, R., Lee, W., & Kwak, S. Y. (2022). A Facile Strategy for Enhancing Tensile Toughness of Poly (Lactic Acid) (PLA) by Blending of a Cellulose Bio-Toughener Bearing a Highly Branched Polycaprolactone. European Polymer Journal, 175. https://doi.org/10.1016/j.eurpolymj.2022.111376.
Zhu, Z., Hu, J., & Zhong, Z. (2022). Preparation and Characterization of Long-Term Antibacterial and pH-Responsive Polylactic Acid/Octenyl Succinic Anhydride-Chitosan@ Tea Tree Oil Microcapsules. International Journal of Biological Macromolecules, 220, 1318-1328. https://doi.org/10.1016/j.ijbiomac.2022.09.038.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.