
A randomized, open labeled, controlled equivalence pilot drug trial to evaluate effect of polyherbal formulations, efficacy and safety of modified panchakarma treatment plan Obetox in patients with Obesity (Medorog)
Dr. Smita Naram 1![]()
![]() , Deepak Mahajan 2
, Deepak Mahajan 2![]()
![]() , Dr. Hemang Parekh 3
, Dr. Hemang Parekh 3![]()
![]()
1 Department
of Research and Development, Ayushakti Ayurveda Pvt Ltd, Bhadran Nagar Cross
Road, Malad, Mumbai-64, India
2 Research
Head, Ayushakti Ayurveda Pvt Ltd, Bhadran Nagar Cross Road, Malad, Mumbai-64,
India
3 Medical Head, Ayushakti Ayurveda Hospital, Bhadran Nagar Cross Road,
Malad, Mumbai-64, India
|
|
ABSTRACT |
||
|
A chronic complex diseases with excessive fat deposition is called as Obesity. It an lead to many metabolic disease like Type II diabetes, heart diseases, thyroid, hypertension, increased bloos cholesterol level, liver disease, sleep apnoea and certain cancers. It can lead to increase the risk of co morbid conditions specially heart diseases. Weight loss can improve or prevent many health issues related to Obesity. Ayurveda says that Obesity is Vikrita Vriddhi (Abnormal increase) of Medodhatu (Fatty Tissue). Aims and Objective- This study was planned to evaluate the efficacy of Ayurvedic treatment Tab Mednil, Tab Suhruday & Obetox Treatment Plan in the treatment of Obesity (Medorog) and to assess the change in various biochemical markers. Method- This is a proof-of-concept study in which a sample size of 60 participants (30 in each group) was considered sufficient to achieve the study objectives. Participants were randomly assigned to two groups according to a computer-generated randomization list. Results- The results show that participants observed a lighter body image, more energy and enthusiasm, and a reduction in obesity-related complications, in terms of weight, H/W ratio, BMI and lipid profile. Conclusion- Obetox protocol along with herbal remedies gives significant
results in the Obesity. |
|||
|
Received 25 May 2024 Accepted 10 October 2024 Published 14 October 2024 Corresponding Author Deepak
Mahajan, drdeepakm@ayushakti.com DOI 10.29121/jahim.v4.i2.2024.60 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Medorog, Obesity, Detox, Virechan, Obetox |
|||
1. INTRODUCTION
A change in diet and an inactive lifestyle have led to an
obesity epidemic in several Asian countries: The consumption of fat and
high-fat foods has increased significantly, while at the same time physical
activity has decreased. With the rapid pace of industrialization and economic
progress, more and more jobs are now sedentary, and dietary habits are also
changing with a decrease in grain consumption and an increase in sugar and fat
intake. All this has led to an increase in obesity and the problems associated
with it. According to a survey conducted by the Nutrition Foundation of India,
45% of women and 29% of men in urban areas of the country are overweight.
Globally, India ranks 7th on the obesity index Nayak (2018). Obesity used to be a
lifestyle issue, but now the World Health Organization has classified it as a
disease. Ayurveda has placed more emphasis on a balanced state of body tissues
in the definition of health.According to Ayurveda, obesity is a condition in
which medodhatu (adipose tissue) is in a state of vikrita vriddhi (abnormal
increase). Definition of Atisthaulya (obesity) A person who is incapacitated
and disfigured with sagging buttocks, abdomen and breasts due to extensive
growth of fat and flesh is called Atisthula (obese) and the condition is termed
as Atisthaulya (obesity) Jadavaji (2001). The term obesity is
defined as an excessive storage of energy in the body in the form of fat.
Obesity is an increase in body weight beyond the limit of skeletal and physical
requirements as a result of excessive accumulation of body fat. A BMI (Body Mass
Index) between 25 and 29.9 kg/m2 is considered overweight, and over 30 kg/m2 is
diagnosed as obesity.In the Charaka Samhita Ibidem Charaka Samhita, the causative factors
for obesity are described as bijadosha (hereditary component) in addition to
dietary, nutritional and psychological factors. Apart from these factors, the
components affecting meda (Fatty Tissue) and kapha (one of the three humors)
can be considered as causative factors for Obesity. Dhatvagni Mandya (weak
digestive fire at the level of body tissues) is the main cause among other
components in the etiopathology of sthaulya Vagbhata, Ashtang Sangraham Sutrasthana Adhyaya 24/15
(1991). In the context of
obesity, the exogenous causes are meda-potentiating diets and cures, while
dosha (three humors), dhatu (body tissues), mala (excretions), srotas (body
channels) etc. belong to the endogenous factors.This small randomized trial was conducted to check the effectiveness
of Ayurvedic treatment Tab Mednil, Tab Suhruday & Obetox Treatment Plan in
the management of Obesity (Medorog) and various biochemical markers.
2. AIMS AND OBJECTIVES
·
Primary
objective:
To evaluate the
efficacy of Ayurvedic treatment Tab Mednil, Tab Suhruday & Obetox Treatment
Plan in the treatment of Obesity (Medorog).
·
Secondary
objectives:
To evaluate the
change in various biochemical markers (Sr. Cholesterol, Sr. Triglycerides, HDL
and LDL) between the two study groups at the end of treatment.
To Evaluation of
the clinical safety of the Ayurvedic treatment packages.
3. METHODOLOGY
Ethical
considerations.
Approval by the
ethics committee.
Informed consent
Written informed
consent was obtained from each participant in the prescribed format prior to
performing any study-related procedures (i.e., physical examination, laboratory
screening, or other investigational procedures) and prior to performing any
study-related procedures. The procedure for obtaining written informed consent
from participants was carried out by the investigator
Study design
Sample size
considerations
As this is a
proof-of-concept study with no previous clinical results available, a sample
size of 60 participants (30 in each group) was considered adequate to meet the
study objectives.
Criteria for
randomization
Participants were
randomized into two groups using a computer-generated randomization list.
Group I: (30 patients) received Tab Mednil, Tab
Suhruday (Metaboost) taken orally twice daily.
Group II: (30 patients) received Tab Mednil, Tab
Suhruday (Metaboost) were taken orally DOSE twice daily along with Obetox
treatment plan (Virechana +Basti)
Study Population
Inclusion Criteria:
1) The patients with generalized obesity (GO)
with BMI ≥ 25 kg/m
2) Patients of either sex between the age
group of 25- 65 years (both years inclusive)
3) Ready to abide by trial procedures and to
give informed consent
Exclusion
criteria:
1) Patients who have genetic history of obesity.
2) Subjects with severe comorbidities, heart disease, stroke, insulin-treated diabetes and chronic renal insufficiency (eGFR & lt; 60 ml/minute/1.73 m 2).
3) Patients current treatment with a weight loss medication (or list under or Phentermine)
4) Patients Impaired mobility or confined to a wheelchair or bed and unable to perform self-care activities.
5) Previous Bariatric surgery in the past is also excluded.
6) Planned bariatric surgery in the next 12 months.
7) Any condition that, in the opinion of the investigator, does not justify the patient’s inclusion in the study.
Study drug-
Each
participant was supplied with the tab Mednil 625 mg thrice a day after food
with lukewarm water at each visit and Tab Suhruday (Metaboost) 510 mg twice a
day after food with lukewarm water at each visit.
Study Duration
Each
participant was in the study for 6 months.
Herbal remedies –
Mednil 625 mg three times daily after food with lukewarm
water and Tab Suhruday (Metaboost) 510 mg twice daily after food with lukewarm
water. All the medicines were purchased from the pharmacy of Ayushakti Ayurveda
Pvt Ltd, Plot Number 78, Stice, Musalgaon, Sinnar, Nashik 422112.
Study Procedures
Potential trial
participants were screened at Ayushakti Ayurveda Pvt Ltd Malad and Borivali
branch, following written informed consent, eligible participants were enrolled
in the study. The trial drugs and obetox treatment was advised according to the
randomization list.
Statistical analysis –
Mean (X), standard
deviation (S.D), standard error (SE), paired TDS test were performed at the
0.05, 0.01 and 0.001 p level. The results were then interpreted as follows:
·
P
>0.05=insignificant.
·
P
<0.05=significant
·
P
<0.01=significant result
·
P
<0.001=highly significant result.
Plan of studies-
Purvakarma (Preoperative)-
Herbal remedies
like Hingashtak churna ¼ tea spoon before food and supachak churna ¼ tea spoon
after food with luke warm water were used for Deepana Pachana (to improve
appetite and digestion).
Rukshana
therapy (Dry therapy) with Sidhdha massage followed by Potali sweda (medicated
bolus) was done on full body for 5 days to improve the superficial circulation
and to lose the Aam (toxins) from superficial strotas.
Snehapan
(internal oleation) with Pachak ghee, Medhya ghee and Mahatriphala ghee was
done till all the features of proper oleation described in ayurvedic texts are
not achieved. During these days full body massage with Mahanarayan oil and
Balada oil was continue to lose the internal toxins at deeper level and bring
them in stomach to remove through anal canal by a procedure of Virechana
(Purgation).
Pradhan Karma (Operative)-
Virechana Karma (detoxification) was done on empty stomach by administering tablets like Virechan and Virechan plus. All expected complications and outcomes were explained to the patient in writing before starting the procedure.
Paschat karma (Post-operative)-
Samsarjan karma (diet plan) was recommended for 3 to 7
days depending on the shuddhi (cleansing signs) during Virechana karma
(detoxification).
4. Results and Discussion
For participation in the study, 98 patients were screened to reach the target of 60 patients. Of these, 21 patients were not included in the study for the reasons indicated in the flowchart. 77 patients were enrolled in the study, 53 of whom completed the study. 25 patients dropped out of the study at various stages of the project, mainly due to poor follow-up. The results show that there was a feeling of lightness and energy, enthusiasm and a reduction in complications of obesity.
The demographic distribution of patients participating in the study was: women n=25 with a mean age of 53.08 years and men n=28 with a mean age of 54.43 years.
Changes in Weight-
The average weight changes were significant in both
groups, but highly significant weight loss was observed in group II, i.e. 13.2
kg in 3 months and 4.54 kg in one month after detoxification. The mean weight
loss after Obetox and Basti (enema) was highly significant compared to that
observed in patients receiving medication only, i.e. 5.6 kg weight loss in 3
months and 2.89 kg in one month. (P-value- <0.0001). The results are shown
in Table 1.
Change in BMI-
The difference in BMI was significant in both groups. The P value was <0.0001 in both groups, yet a BMI difference of 5.03 was found in group II compared to 2.2 in group I. The results are shown in Table 1.
Waist
Hip ratio-
Highly
significant difference was found in the waist and Hip ratio in both the groups.
5.03 cms difference was found in group II and 2.27 cms difference was found in
group I in waist and Hip ratio. The results are mentioned in table-1.
Serum Cholesterol levels-
In both the
groups’ highly significant difference was observed in serum Cholesterol levels.
In group II Obetox programme along with medicines gives better result in serum
cholesterol levels as compared with Group I. The results are mentioned in
table-1.
Serum Triglyceride level-
Highly
significant difference was found in both the groups. Still serum triglyceride
was better reduced with Obetox and medicines P value 0.0044 as compared to
group with only medicines P value 0.008. The results are mentioned in table-1.
Chart 1
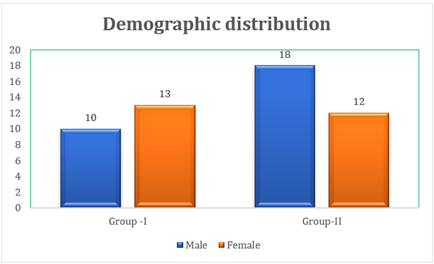
|
Chart 1 Demographic Distribution |
Table 1
|
Table 1 Changes
in the body parameters and lipid profile. |
||||||||
|
Difference in Weight (In Kg) |
||||||||
|
BT |
V2 |
AT |
Diff in 1 month |
Mean (In 3 months) |
SD |
SEM |
P value |
|
|
Group-1 (n=23) |
74.86 |
71.96957 |
69.21304 |
2.89043478 |
-5.648 |
3.014 |
0.6284 |
<0.0001 |
|
Group-II(n=30) |
74.48 |
69.94 |
66.24 |
4.54 |
-13.2 |
3.26 |
0.5952 |
<0.0001 |
|
Difference in BMI |
||||||||
|
BT |
V2 |
AT |
Diff in 1 month |
Mean (In 3 months) |
SD |
SEM |
P value |
|
|
Group-1 (n=23) |
30.47 |
29.30042 |
28.18866 |
1.16542678 |
-2.278 |
1.252 |
0.2612 |
<0.0001 |
|
Group-II(n=30) |
28.39 |
26.11832 |
23.36326 |
2.27571473 |
-5.03 |
1.225 |
0.2236 |
<0.0001 |
|
Difference in W/H Ratio |
||||||||
|
BT |
V2 |
AT |
Diff in 1 month |
Mean (In 3 months) |
SD |
SEM |
P value |
|
|
Group-1 (n=23) |
0.921 |
0.921712 |
0.921588 |
-0.0005135 |
-2.278 |
1.252 |
0.2612 |
<0.0001 |
|
Group-II(n=30) |
0.963 |
0.96571 |
0.96456 |
-0.0027374 |
-5.03 |
1.225 |
0.2236 |
<0.0001 |
|
Difference in Cholesterol level |
||||||||
|
BT |
V2 |
AT |
Diff in 1 month |
Mean (In 3 months) |
SD |
SEM |
P value |
|
|
Group-1 (n=23) |
181.5 |
157.4739 |
137.4391 |
24.0652174 |
-44.1 |
46.04 |
9.6 |
0.0001 |
|
Group-II(n=30) |
187.1 |
156.49 |
131.2167 |
30.5933333 |
-55.87 |
45.38 |
8.285 |
<0.0001 |
|
Difference in Triglycerides level |
||||||||
|
BT |
V2 |
AT |
Diff in 1 month |
Mean (In 3 months) |
SD |
SEM |
P value |
|
|
Group-1 (n=23) |
137 |
124.7087 |
117.9565 |
12.273913 |
-19.03 |
28.71 |
5.987 |
0.0044 |
|
Group-II(n=30) |
168.7 |
138.9433 |
109.13 |
29.7833333 |
-59.6 |
114.5 |
20.91 |
0.008 |
|
Difference in LDL level |
||||||||
|
BT |
V2 |
AT |
Diff in 1 month |
Mean (In 3 months) |
SD |
SEM |
P value |
|
|
Group-1 (n=23) |
138.9 |
116.7304 |
108.2348 |
22.1347826 |
-30.63 |
24.52 |
5.113 |
<0.0001 |
|
Group-II(n=30) |
125 |
96.85667 |
90.22333 |
28.1173333 |
-34.75 |
38.37 |
7.005 |
<0.0001 |
|
Difference in HDL level |
||||||||
|
BT |
V2 |
AT |
Diff in 1 month |
Mean (In 3 months) |
SD |
SEM |
P value |
|
|
Group-1 (n=23) |
48.77 |
49.02609 |
50.95217 |
-0.2565217 |
-2.183 |
8.472 |
1.766 |
0.2296 |
|
Group-II(n=30) |
47.37 |
48.75333 |
50.23333 |
-1.3866667 |
-2.86667 |
11.12 |
2.031 |
0.008 |
5. Discussion
Charakacharya has given a
detailed description of Obesity in his Charaksamhita under the
heading Medorog, which contains details of causative factors,
etiopathogenesis, signs and symptoms. Acharya Sushruta has described the
complications of obesity in his Sushruta Samhita.
Nidan parivarjan (to avoid causative factors) is the first
line of treatment stated by the Charaka.
Obesity (Medorog) can be treated with
two major therapies Shodhana and Shamana. In Shodhana bio purification therapies are used like Vamana (Medicated emesis) and Virechana (Medicated purgation) and are
advisable according to the Dehbala
(strength of the patient) and Vyadhibala (strength
of the disease). Virechana karma normalizes the agni (digestive fire) and
brings the tridosha in balance state also it clears the Srotavarodha
(obstruction in micro channels) Acharya (2008). Panchakarma is a specially designed five procedures of
bio-purification of the entire body. These procedures bring the biological
system into normal homeostasis, rejuvenate the body and also help to achieve
the expected pharmacotherapeutic effect of herbal medicines Chaturvedi (2019).
During the treatment, Rukshana therapy (dry therapy) with
Sidhdha massage followed by Potali sweda (medicinal bolus) was performed on the
whole body, which can lead to an increase in lymphatic drainage, and lymphatic
massage has been shown to help in water loss and ultimately weight loss Lulla & Prasad (2013). During the virechana
process, cellular fluid is drained through the interstitial fluid into the
vascular compartment and from there into the gastrointestinal tract for removal
through the anal canal. This means that this process causes a biochemical change
by modulating the fluid compartments Acharya (2008), Arthur & John (n.d.). Cuminum cyminum (Shwet
jirak) contains essential fatty acids, volatile oils, phenolic compounds
therefore it has an anti-obesity effect and it has effect on weight, BMI, waist
circumference and H/W ratio Mohseni
et al. (2021). Cuminum cyminum (Shwet jirak) is
associated with significant decrease in serum insulin level hence has an impact
on weight and BMI Taghizadeh et al. (2015). Enicostemma
littorale blume (Mamejava) has alkaloids which reduces weight, BMI, serum
lipids hence possesses anti-lipase and anti-obesity potential Garg & Singh (2015). Ciccus
quadrangularis (Gokshur) have significant anti hyperlipidaemic effect Talreja
(2015). Trikatu is a polyherbal combination contains
Maricha (Piper nigrum), Pippali (Piper longum), Shunthi (Zinziber officinalis)
is mostlyused in all the medicines used in Obesity (Medorog) as it has
anti-obesity and anti hyperlipidaemic effect Thakkar & Jadhav (2022). Guggulu resin
significantly decreases weight and ultimately BMI and obesity it also reduces
serum cholesterol, triglycerides, and LDL level Gupte et al. (2020).
6. Conclusion
Causative factors for Obesity (Medorog) mentioned in the ayurveda texts are changing now a days. Stress, altered faulty food habit, lack of exercise, sedentary life style is becoming prominent causative factors. The result indicates that both Virechana Karma and the preparatory procedures to correct lipid metabolism have an effect against the metabolic syndrome caused by insulin resistance and reduce body weight, BMI, serum triglycerides and blood glucose levels. Obetox protocol along with herbal remedies gives significant results in the Obesity.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Acharya, Y.T. (2008). Charaka
Samhita of Agnivesha, Charaka, Sutra Sthana, Adhyay 1, Verse 17, Reprint
Edition. Varanasi: Chaukhamba Sanskrit Sansthan, 680.
Acharya, Y.T. (2008). Charaka
Samhita of Agnivesha, Charaka, Sutra Sthana Adhyay 23, Verse 14, Reprint
Edition. Varanasi: Chaukhamba Sanskrit Sansthan, 127.
Arthur, G., & John, H. (n.d.). Textbook of Medical Physiology. Philadelphia, Pennsylvania: Elsevier Saunders, 1066, 811.
Chaturvedi, A. (2019). A Clinical Study on Virechana Karma (Therapeutic Purgation) Over the Gut Flora with Special Reference to Obesity, 40(3), 179-184. https://doi.org/10.4103/ayu.AYU_302_19
Garg, A., & Singh, R. (2015). Antiobesity Activity of Ethanolic Extract of Cassia Auriculata in High Fat Diet Induced Obese Rats. Internation Journal of Pharm Science, 7(4), 237-43. https://doi.org/10.22159/ijpps.2015v7i4.4275
Gupte, P., Harke, S., Deo, V., Shrikhande, B.B., Mahajan, M., & Bhalerao, S. (2020). A Clinical Study To Evaluate The Efficacy of Herbal Formulation For Obesity (HFO-02) In Overweight Individuals. Journal of Ayurveda and Integrative Medicine, 11(2), 159-162. https://doi.org/10.1016/j.jaim.2019.05.003
Ibidem Charaka Samhita, Sutrasthana Adhyaya 21 Verse 3, 116.
Jadavaji, T. (2001). Agnivesha,
Charaka, Dridhabala, Charaka Samhita Sutrasthana Adhyaya 21/4, (Ed. 5th),
Chaukhambha Sanskrit Sansthana, Varanasi, 116.
Lulla A., & Prasad, U.N. (2013). An Open Comparative Randomized Pragmatic Clinical Trial Evaluating Efficacy of Virechana in Sthoulya. Bangalore: RGUHS, 189.
Mohseni, F., Ahmadiani, E. S., & Hekmatdoust, A. (2021). The Effect of Cumin on Anthropometric Measurements: A Systematic Review of Randomized Controlled Clinical Trials. Obesity Medicine, 23. https://doi.org/10.1016/j.obmed.2021.100341
Nayak, T. K. (2018). Role of Maintaining Diet in Life Style Disorder Wsr to Obesity. International Journal of Research in Medical Sciences and Technology, (IJRMST), 6.
Taghizadeh, M., Memarzadeh, M. R., Asemi, Z., & Esmaillzadeh, A. (2015). Effect of the Cumin Cyminum L. Intake on Weight Loss, Metabolic Profiles and Biomarkers of Oxidative Stress in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Annals of Nutrition and Metabolism, 66(2-3), 117-124. https://doi.org/10.1159/000373896
Talreja, T. (2015). A Systematic
Review on Evidence Based Validation of Two Medicinal Plants for the Safe and
Efficient Management of Obesity. World Journal of Pharmaceutical Research,
4(10), 2657-2669.
Thakkar, S., & Jadhav, M. (2022). A Review on Formulations of Trikatu Choorna in Management of Sthaulya
(Obesity) Mentioned in Bharat Bhaishajya Ratnakara.
Vagbhata, Ashtang Sangraham Sutrasthana Adhyaya 24/15 (1991). (Ed.1st), 294, CCRAS, New Delhi.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.