
Nature's Shield: Unveiling the Protective Power of Sharkara Yukta Gau-Dugdha as Prativisha against Datura metel Induced Toxicity in Albino Wistar Rats - A Research Article
Dr. Nidhi Porwal 1![]()
![]() , Anup Kumar
Gakkhar 2
, Anup Kumar
Gakkhar 2![]()
![]() , Dr. Ramesh Chandra
Tiwari 3
, Dr. Ramesh Chandra
Tiwari 3![]()
![]() , Dr. Bhawana
Mittal 4
, Dr. Bhawana
Mittal 4![]()
![]() , Dr. Bhanu
Gupta 5
, Dr. Bhanu
Gupta 5![]()
![]()
1 Post
Graduate Scholar, Rishikul Campus Haridwar, Department of Agad Tantra Evum
Vidhi Vaidyak, India
2 Registrar
of Uttarakhand Ayurveda University, India
3 Professor and H.O.D, Department of Agad Tantra Evum Vidhi Vaidyak,
India
4 Assistant Professor, Department of Agad Tantra Evum Vidhi Vaidyak,
India
5 Post Graduate Scholar, Vaidya Yagya Dutta Ayurvedic College and
Hospital, Department of Shalya Tantra, India
|
|
ABSTRACT |
||
|
Datura (Datura
metel) is recognized as a cerebrotoxic deliriant poison and is concurrently
categorized as "Upavisha" within Ayurvedic
principles. Ayurveda encompasses a spectrum of toxic substances along
with their respective antidotes; however, the mechanisms underlying these
antidotal effects remain unelaborated in the ancient texts. A segment in the
"Rasa jal Nidhi" Part 3 (Chapter 8), titled "Dhusturbeejasya
Shanti," delineates the potential antidote properties of Gau
dugdha in a proportion of one prastha and sharkara (sugar)
in a quantity of two pal, positing a potential counteraction against Datura
toxicity. The objective of this study is to scientifically evaluate the
efficacy of these antidotal agents in mitigating Datura seed-induced
toxicity, so that it can be useful in emergencies. Methods: Albino Wistar
rats are employed as the chosen animal model to investigate the antidotal
effect of Sharkara Yuka Gau-dugdha against Datura beeja (seed)
powder-induced toxicity. The assessment encompasses a comprehensive range of
criteria encompassing alterations in weight, modifications in dietary and
hydration habits, behavioral shifts, lethaginss, fluctuations in temperature,
emergence of convulsive episodes, performance in the Radial Arm Test, Rotarod
activity, locomotion, and mortality. Results: Following administration of Sharkara
Yuka Gau-dugdha, discernible alleviation of the deleterious effects
associated with Datura exposure is evident. Noteworthy observations
encompass a reduction in responses during the Radial Arm Test, suggestive of
a positive influence on cognitive faculties such as learning and memory.
Additionally, an increase in time spent on the Rotarod apparatus signifies
enhanced muscle strength and augmented motor function. Conclusion: The findings
gleaned from this experimental study culminate in the conclusion that Sharkara
Yuka Gau-dugdha holds moderate potential to ameliorate the extent of
toxic manifestations elicited by Datura exposure. |
|||
|
Received 07 August 2023 Accepted 23 November 2023 Published 20 December 2023 Corresponding Author Dr. Nidhi
Porwal, gupta.nidhi593@gmail.com DOI 10.29121/jahim.v3.i2.2023.33 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Datura, Sharkara, Gau- Dugdha, Agad, Toxicity,
Antidote |
|||
1. INTRODUCTION
In antiquity, Ayurveda held a preeminent status as an advanced field of knowledge. Within its realm, "Agad tantra" pertains to the branch that addresses the toxic effects of various creatures such as snakes, spiders, insects, and rodents, along with their corresponding treatments. The term "Gada" signifies poison, and the counteragents employed are termed "Agada." In contemporary toxicology, the discipline engages in the comprehensive study of toxins, encompassing their origins, properties, modes of action, resulting symptoms, lethal doses, fatal intervals, and approaches to detection, quantification, and post-mortem findings. Datura (Datura metel), classified as cerebrotoxic and deliriant, is acknowledged within the Ayurvedic canon as an "Upavisha." This genus comprises toxic herbs and shrubs that attain heights of 3-4 feet. Notable for their intoxicating and narcotic attributes, these plants induce temporary insensibility in moderate doses, eliciting stupefying effects. Datura metel, commonly known as Devil's Trumpet or Angel's Trumpet, is a fascinating and enigmatic plant that has captured human fascination for centuries. Belonging to the Solanaceae family, this flowering plant is renowned for its striking and trumpet-shaped blossoms, which exude an alluring fragrance in the twilight hours. Throughout history, Datura metel has played a significant role in various cultures and societies. Its ethereal appearance, coupled with its potent psychoactive properties, has led to a myriad of religious, spiritual, and medicinal applications. However, it's important to note that Datura metel is a highly toxic plant and should be handled with extreme caution. Datura metel, along with other species in the Datura genus, is renowned for its extreme toxicity. The plant contains a cocktail of potent alkaloids, including atropine, scopolamine, and hyoscyamine, which are responsible for its psychoactive effects as well as its danger to human health. These tropane alkaloids act as competitive antagonists of acetylcholine receptors in the nervous system, leading to a wide range of physiological and psychological effects. Ingestion of any part of the Datura metel plant can have severe consequences, and even small amounts of the plant can be lethal. The toxicity levels can vary between different plant parts and growth stages, with the seeds, flowers, and leaves often carrying the highest concentration of alkaloids. Symptoms of Datura toxicity usually appear within 30 minutes to a few hours after ingestion and can last for several days. The effects are highly unpredictable and may vary from person to person. One of the most distinctive effects of Datura toxicity is vivid and often terrifying hallucinations. These hallucinations can be auditory, visual, or tactile, leading to confusion and disorientation. Datura poisoning can induce a state of extreme delirium, making it challenging for the affected individual to distinguish between reality and hallucinations. Datura alkaloids disrupt the autonomic nervous system, leading to dry mouth, dilated pupils, and blurred vision. The tropane alkaloids in Datura can cause tachycardia (increased heart rate), which can be particularly dangerous for individuals with pre-existing heart conditions. Datura toxicity can result in a loss of motor coordination, making simple tasks difficult and potentially leading to accidents. In severe cases, Datura poisoning may trigger seizures, posing a significant risk to the individual's health and safety. In extreme cases or when consumed in high doses, Datura toxicity can lead to a coma and even death due to respiratory failure or cardiac arrest. It's important to note that intentional use of Datura for its hallucinogenic effects is not only illegal but also extremely dangerous. The risk of overdose and unpredictable reactions make it a highly risky choice for recreational purposes. Additionally, accidental poisoning can occur, especially in children or individuals unaware of the plant's toxicity. Various poisons have been described in Ayurveda 6-8 along with their antidotes. These antidotes are readily available in nature. Mode of action of these antidotes is not mentioned in texts. In Ayurvedic literature according to Ras jal nidhi part 3 (chaptar 8) under the heading of dusturbeejasya shanti, Gau dugdha-one prastha and sharkara-2 pal has been described to be possessing antidote action which may act by some way to counter toxicity of Datura.9-11 The properties of gau dugdha, according to Ayurveda are Madhura, Sheeta, Mridu, Snigdha, Bahal, Slakshana, Picchila, Guru, Manda, Prasanna, Ojovardhaka and Pravara Jivaniya. Thus, properties of the milk are similar to Ojas i.e, promoting immunity or body defense mechanism. The properties of Ojas are opposite to Visha. Sharkara contains madhura rasa, snigdha guna, sita virya and madhura vipaka. Sharkara is chaksusya, dhatu vardhaka, hridya, pittahara, vatahara and vrisya. Sharkara pacifies vata, pitta, rakta dosha and cures the symptoms caused by unconsiousness, vomiting and poison intake. Main chemical constituents of Gau-dugdha are Protein (3.2%), Fat (3.9%), Carbohydrates i.e. Lactose (4.9%), Mineral compounds (0.90%), Water (87%), trace of Amino acids (101.7mg/litre), Minerals, Enzymes, Vitamins, Calories-65% and sharkara are Energy: 1,619 kJ (387 kcal), Water: 0.03 g, Iron: 0.01 mg, Carbohydrates: 99.98 g, Riboflavin (Vit. B2): 0.019 mg (2%), Calcium: 1 mg, Sugars: 99.91 g. It is necessary to verify the efficacy of these antidotes on scientific parameters15 so that it can be useful in emergencies. Hence present study entitled “Sharkara Yukta Gau-dugdha as an Antidote in Datura poisoning in albino wistar rats, was carried out to establish the action and mechanism of Sharkara Yukta Gau-dugdha against toxic effects of Datura on albino wistar rats. Acharya (2000)
2. MATERIAL AND METHODS
2.1. DRUG IDENTIFICATION AND AUTHENTICATION
The sample of raw drugs were authenticated by expert of Dravyaguna department U.A.U. Rishikul campus, Haridwar.

2.2. COLLECTION OF DRUGS
· Date of collection (Datura fruits) - June 2022
Fresh Datura fruits were collected from Herbal garden, Shyampur, Nazibabad road, Haridwar. Fruits were dried in sunlight and seeds were collected for the study. Gau dugdha and Sharkara were collected from the authentic sources.

·
Drug
Preparation-
Coarse powder of Datura seeds was prepared by grinding and mixed with 1% CMC solution.

Animal Experiment Material for In Vivo study Albino wistar rats of age 8-12 weeks and weight ranges 100-250 gm were procured. Poisoning was induced by Datura seeds and test drug was Gau dugdha and Sharkara.

Determination of acute toxicity of Datura seeds is performed as per guideline of OECD 423 in Albino wistar rats.
Determination of protective action of Sharkara Yukta Gau-dugdha against toxicity of Datura seed Reduction in mortality and change in behaviour of rats due to a lethal dose of Datura seed after administration of Sharkara Yukta Gau-dugdha are determined.
2.3. STUDY DESIGN
1) Animal Sources:
· Healthy rats were selected for experimentation.
· Animals were maintained as per Animal Ethical Committee regulations.
· Experimental protocol was approved from IAEC (Institutional Animal Ethics Committee) and whole experiment was performed under the guidelines of CPCSEA (Committee for Purpose of Control and Supervision of Experimental on Animals).
2) Inclusion criteria-
Healthy Female wistar rats weighing between 100-250 grams.
3) Exclusion criteria-
Unhealthy rats were discarded.
The rats were discarded, if the weight is not within ±20% of the mean initial weight.
The parous and the pregnant female rats were discarded.
Rats which were under trial of other experiment.
Table 1
|
Table 1 Showing Animal Experiment Groups |
||||
|
S.
No. |
GROUPS |
GROUP
NAME |
TREAMENT |
ANIMALS |
|
1. |
Group 1 |
Control Group |
No treatment |
6 |
|
2. |
Group 2 |
Acute Poisoning Group |
Oral administration
of powder of datura seeds |
6 |
|
3. |
Group 3 |
Study Group |
Sharkra
yukta Gau–dugdha after administration
of datura seeds |
6 |
4) Housing and feeding conditions- The temperature in the experimental animal room had been 220C (±30C). Although the relative humidity had been at least 30% and preferably not exceed the sequence being 12 hours light, 12 hours dark. For feeding, conventional laboratory diets may be used with an unlimited supply of drinking water.

5) Oral Acute Toxicity study-:
The antidote study was performed as per OECD423 guideline-
Animal species used - Wister rats
Sex of animal - Female
Age of animal - 8-12 weeks
Average weight of animal - 100-250 gm
Total no. of animals - 18
No. of groups - 3
No. of animal in each group - 6
Period of fasting - Over night
Feeding - Standard palatable diet
Water - R.o. drinking water
Temperature - 22±3˚C
Humidity - 30-70%
Vehicle - CMC solution
Route - Oral
Experimental drug - Datura beeja (seed)
Antidotal drug - Sharkara yukta gau-dugdha
Observation period - 14 days
6) Preparation of animals-
The animals are randomly selected, marked with Picric acid, H (mark on head), B (Mark on Back), T (mark on Tail) for individual identification, and kept in their cages for at least 5 days prior to dosing to allow for acclimatisation to the laboratory conditions.

7) Number of animals and dose levels-
Eighteen albino wistar rats had been divided in three groups. Each group contain 6 wistar rats as follows-
Group 1 had six wistar rats and they had received CMC (1%) solution 5ml/kg/P.O in a single dose.
Group 2 had six wistar rats and they had received Datura beej (seed) churna 111mg/ kg/P.O (10%) in a single dose along with C.M.C. solution.
Group 3 had six wistar rats and they had received Sharkara yukta gau-dugdha (8.9:1.1) 10ml/kg orally after inducing poisoning by Daturabeej (seed) churna 111mg/ kg/P.O (10%) in a single dose.
Dose was administered orally in a single dose by gavage.

Dose was calculated according to their body weight.
Dose was decided on the basis of Pagets & Brane's rule, 1964 i.e. (0.018× human dose ×5).
Following the period of fasting, the animals were weighed and the test substance administered.
After the substance had been administered, food was withheld for a further 3-4 hours in rats.
Dose Calculation for Albino wistar rats the conversion factor from man to rats is 0.018 so according to this, all the doses were calculated. Dose for rats was obtained from the following formula Dose of rats = 0.018 × human dose × 5.
Dose Calculation for Datura Seeds, Human fatal Dose for Datura Seeds is considered as 120 crushed seeds i.e. calculated as 1234 mg. According to the conversion factor, fatal dose in albino wistar rats for Datura is 111 mg Hence per kg wt. Fatal dose for albino wistar rats = 0.018 x 1234 x 5 = 111 mg/kg.
Dose Calculation for Sharkara Yukta Gau-dugdha: Human therapeutic dose of Gau-dugdha and Sharkara is considered as 768 gm and 96 gm. The ratio of Gau-dugdha and Sharkara for albino wistar rats is 8.9:1.1. Dose Calculation for Sharkara Yukta Gau-dugdha, according to the wt. of albino wistar rats is 10ml/kg.

Table
2
|
Table 2 Showing Dosing Chart of Group 1 |
||
|
Group 1 |
||
|
Marking |
Weight (gm) |
CMC Sol 5 ml/ kg (ml) |
|
H |
200 |
1 |
|
B |
195 |
0.975 |
|
T |
145 |
0.725 |
|
HB |
196 |
0.98 |
|
BT |
180 |
0.9 |
|
HT |
172 |
0.86 |
Table
3
|
Table 3 Showing Dosing Chart of Group 2 |
|||
|
Group 2 |
|||
|
Marking |
Weight (gm) |
Dhatura seeds 111 mg/ kg (10 %) |
|
|
Dose in mg |
Dose in ml |
||
|
H |
171 |
18.981 |
0.19 |
|
B |
196 |
21.756 |
0.22 |
|
T |
174 |
19.314 |
0.19 |
|
HB |
217 |
24.087 |
0.24 |
|
BT |
165 |
18.315 |
0.18 |
|
HT |
152 |
16.872 |
0.17 |
Table
4
|
Table 4 Showing Dosing Chart of Group 3 |
||||
|
Group 3 |
||||
|
Marking |
Weight (gm) |
Dhatura seeds 111 mg/ kg (10 %) |
Cow milk + suger (8.9:1.1) 5 ml/kg |
|
|
Dose in mg |
Dose in ml |
Dose in ml |
||
|
H |
134 |
14.874 |
0.15 |
0.67 |
|
B |
180 |
19.98 |
0.20 |
0.9 |
|
T |
190 |
21.09 |
0.21 |
0.95 |
|
HB |
213 |
23.643 |
0.24 |
1.065 |
|
BT |
220 |
24.42 |
0.24 |
1.1 |
|
HT |
193 |
21.423 |
0.21 |
0.965 |
8) Observations- Animals are observed individually after dosing at least once during the first 30 minutes, periodically during the first 24 hours, with special attention given during the first 4 hours, and daily thereafter, for a total of 14 days.
All observations were systematically recorded with individual records being maintained for each animal.
Observations include changes in skin and fur, eyes, mucous membranes, salivation, Lethargy, sleep, coma, convulsions, tremors, diarrohea, morbidity, mortality, water and food intake, rectal temperature, Learning memory observation on radial arm maze was observed.
3. ASSESMENT CRITERIA
The assessment criteria of antidotal effect of Sharkara yukta Gau-dughdha were made on the basis of-
· Weight changes
· Changes in food and water habits
· Behaviour changes-Salivation, Urination
· Change in temperature
· Appearance of Convulsion
· Radial Arm Test
· Rotarod Test
· Mobility & Mortality
4. RESULTS AND DISCUSSION
The results are expressed as Mean ± SEM Comparison between each group were performed by analysis of variance (ANOVA) with Dunnett's 't’ test for determining the level of significance. P value less than 0.05 was considered as statistically significant. Acharya (2012)
Table 5
|
Table 5 Showing Statistical Analysis of Weight Changes |
||||||
|
Weight (gm) |
Group 1 |
Group 2 |
Group 3 |
|||
|
Mean±SEM |
No |
Mean±SEM |
No |
Mean±SEM |
No |
|
|
0 day |
181.33±8.48 |
6 |
179.17±9.57 |
6 |
188.33±12.46 |
6 |
|
8 day |
185.05±8.44 |
6 |
185.95±12.34 |
5 |
194.11±12.43 |
6 |
|
14day |
189.03±8.34 |
6 |
188.96±12.14 |
5 |
200.75±12.91 |
6 |
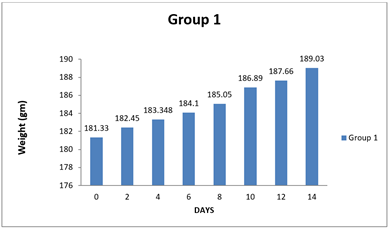
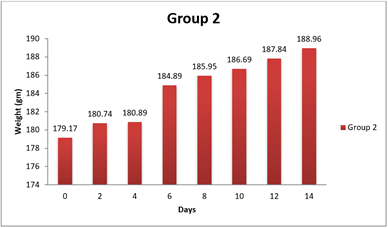
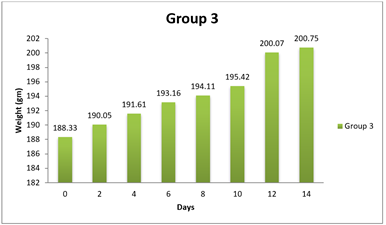
4.1. Result of Weight changes
Table 6
|
Table 6 Showing Comparison Between Weight (gm) Changes of all groups |
||||
|
Dunnett's multiple
comparisons test |
Mean Diff. |
95.00% CI of diff. |
Significant? |
Adjusted P Value |
|
0
day:Group 1 vs. 0 day:Group 2 |
2.160 |
-42.95
to 47.27 |
No |
0.9998 |
|
0
day:Group 1 vs. 0 day:Group 3 |
-7.000 |
-52.11
to 38.11 |
No |
0.9994 |
|
0
day:Group 1 vs. 8 day:Group 1 |
-3.720 |
-48.83
to 41.39 |
No |
0.9997 |
|
0
day:Group 1 vs. 8 day:Group 2 |
-4.620 |
-51.93
to 42.69 |
No |
0.9996 |
|
0
day:Group 1 vs. 8 day:Group 3 |
-12.78 |
-57.89
to 32.33 |
No |
0.9949 |
|
0
day:Group 1 vs. 14day:Group 1 |
-7.700 |
-52.81
to 37.41 |
No |
0.9993 |
|
0
day:Group 1 vs. 14day:Group 2 |
-7.630 |
-54.94
to 39.68 |
No |
0.9993 |
|
0
day:Group 1 vs. 14day:Group 3 |
-19.42 |
-64.53
to 25.69 |
No |
0.9263 |

There were no significant differences observed between the
weight changes of Group 1 and the other groups at various time points (0 day, 8
day, and 14 day). The mean differences in weight changes between Group 1 and
the other groups were relatively small and not statistically significant, as
evidenced by the wide 95.00% confidence intervals that include zero. The
adjusted p-values for all comparisons were close to 1, indicating that the
differences observed were likely due to chance rather than meaningful effects.
Therefore, based on this analysis, there were no significant differences in
weight changes between the groups during the study period. Paradakara (2011)
Table 7
|
Table 7 Showing Statistical Analysis of Food Consumption |
||||||
|
Food consumption (gm) |
Group 1 |
Group 2 |
Group 3 |
|||
|
Mean± SEM |
No |
Mean±SEM |
No |
Mean±SEM |
No |
|
|
0
day |
20±0.00 |
6 |
20±0.00 |
6 |
15±0.00 |
6 |
|
8
day |
25±0.00 |
6 |
20±0.00 |
5 |
15±0.00 |
6 |
|
14day |
25±0.00 |
6 |
20±0.00 |
5 |
25±0.00 |
6 |
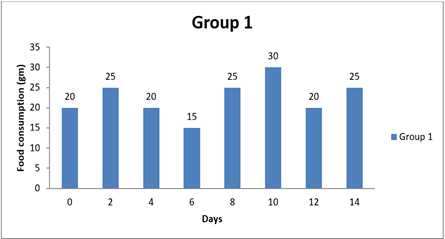
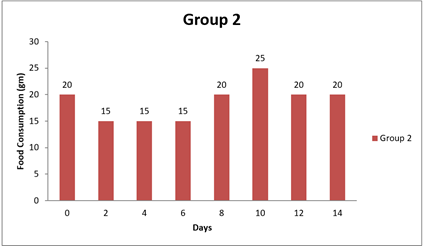
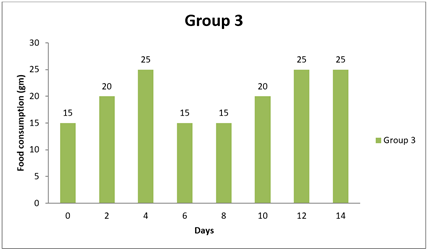
4.2. Result of Food Consumption
Table 8
|
Table 8 Showing Comparison Between Food Consumption of all Groups |
||||
|
Dunnett's multiple
comparisons test |
Mean Diff. |
95.00% CI of diff. |
Significant? |
Adjusted P Value |
|
0
day:Group 1 vs. 0 day:Group 2 |
15.35 |
15.35
to 15.35 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 0 day:Group 3 |
5.000 |
5.000
to 5.000 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 8 day:Group 1 |
-5.000 |
-5.000
to -5.000 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 8 day:Group 2 |
15.44 |
15.44
to 15.44 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 8 day:Group 3 |
5.000 |
5.000
to 5.000 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 14day:Group 1 |
-5.000 |
-5.000
to -5.000 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 14day:Group 2 |
13.15 |
13.15
to 13.15 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 14day:Group 3 |
-5.000 |
-5.000
to -5.000 |
Yes |
<0.0001 |
Significant differences were observed in food consumption between Group 1 and the other groups at various time points (0 day, 8 day, and 14 day). The mean differences in food consumption between Group 1 and the other groups were statistically significant, as evidenced by the narrow 95.00% confidence intervals that do not include zero. The adjusted p-values for all comparisons were very small (less than 0.0001), indicating strong evidence of significant differences in food consumption between the groups during the study period. Specifically, Group 1 showed higher food consumption than Groups 2 and 3 at 0 day and 8 day, as indicated by positive mean differences. Conversely, at 8 day and 14 day, Group 1 had lower food consumption than Groups 1, 2, and 3, as indicated by negative mean differences. Overall, this analysis suggests that there are significant differences in food consumption between the groups, with Group 1 exhibiting distinct patterns of food intake compared to the other groups throughout the study duration.
Table 9
|
Table 9 Showing Statistical Analysis of Water Consumption |
||||||
|
Water consumption (ml) |
Group 1 |
Group 2 |
Group 3 |
|||
|
Mean±SEM |
No |
Mean±SEM |
No |
Mean±SEM |
No |
|
|
0
day |
4.56±0.00 |
6 |
4.65±0.00 |
6 |
5.69±0.00 |
6 |
|
8
day |
4.85±0.00 |
6 |
4.56±0.00 |
5 |
6.69±0.00 |
6 |
|
14day |
5.93±0.00 |
6 |
6.85±0.00 |
5 |
6.19±0.00 |
6 |
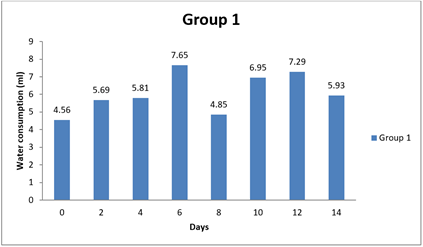
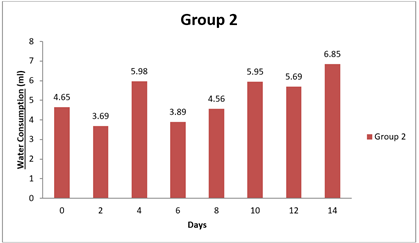
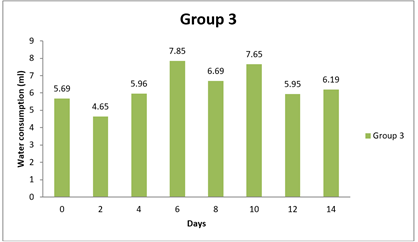
4.3. Result of Water Consumption
Table 10
|
Table 10 Showing Comparison Between Water Consumption (ml) of all Groups |
||||
|
Dunnett's multiple
comparisons test |
Mean Diff. |
95.00% CI of diff. |
Significant? |
Adjusted P Value |
|
0
day:Group 1 vs. 0 day:Group 2 |
-15.44 |
-15.44
to -15.44 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 0 day:Group 3 |
-1.130 |
-1.130
to -1.130 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 8 day:Group 1 |
-0.2900 |
-0.2900
to -0.2900 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 8 day:Group 2 |
-15.44 |
-15.44
to -15.44 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 8 day:Group 3 |
-2.130 |
-2.130
to -2.130 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 14day:Group 1 |
-1.370 |
-1.370
to -1.370 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 14day:Group 2 |
-15.44 |
-15.44
to -15.44 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 14day:Group 3 |
-1.630 |
-1.630
to -1.630 |
Yes |
<0.0001 |
Significant differences were observed in water consumption between Group 1 and the other groups at various time points (0 day, 8 day, and 14 day). The mean differences in water consumption between Group 1 and the other groups were statistically significant, as evidenced by the narrow 95.00% confidence intervals that do not include zero. The adjusted p-values for all comparisons were very small (less than 0.0001), indicating strong evidence of significant differences in water consumption between the groups during the study period. Specifically, Group 1 showed lower water consumption compared to Groups 2 and 3 at 0 day and 8 day, as indicated by negative mean differences. Similarly, at 8 day and 14 day, Group 1 had lower water consumption than Groups 1, 2, and 3, as indicated by negative mean differences. Overall, this analysis suggests that there are significant differences in water consumption between the groups, with Group 1 consistently exhibiting lower water intake compared to the other groups throughout the study duration.
Table 11
|
Table 11 Showing Statistical Analysis of Rotarod Test |
||||||
|
Rotarod (Sec) |
Group 1 |
Group 2 |
Group 3 |
|||
|
Mean±SEM |
No |
Mean±SEM |
No |
Mean±SEM |
No |
|
|
0 day |
9.53±1.13 |
6 |
6.09±1.55 |
6 |
6.11±0.71 |
6 |
|
8 day |
12.98±1.39 |
6 |
8.02±0.65 |
5 |
11.26±1.26 |
6 |
|
14day |
17.49±1.41 |
6 |
8.09±0.77 |
5 |
15.06±0.95 |
6 |
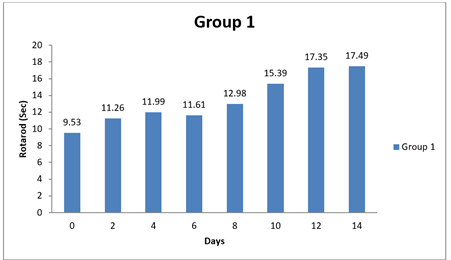
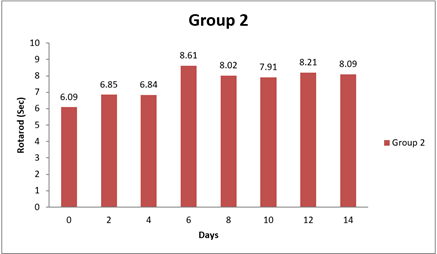
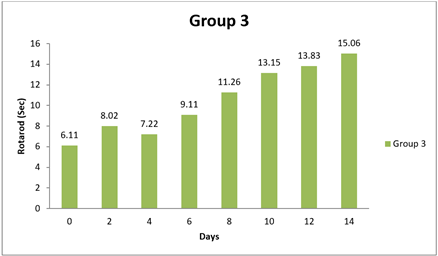
4.4. Result of Rotarod
Table 12
|
Table 12 Showing Comparison Between Rotarod (Sec) Test of all Groups |
||||
|
Dunnett's multiple
comparisons test |
Mean Diff. |
95.00% CI of diff. |
Significant? |
Adjusted P Value |
|
0
day:Group 1 vs. 0 day:Group 2 |
3.440 |
-1.340
to 8.220 |
No |
0.3286 |
|
0
day:Group 1 vs. 0 day:Group 3 |
3.420 |
-1.360
to 8.200 |
No |
0.3362 |
|
0
day:Group 1 vs. 8 day:Group 1 |
-3.450 |
-8.230
to 1.330 |
No |
0.3250 |
|
0
day:Group 1 vs. 8 day:Group 2 |
1.510 |
-3.503
to 6.523 |
No |
0.9944 |
|
0
day:Group 1 vs. 8 day:Group 3 |
-1.730 |
-6.510
to 3.050 |
No |
0.9842 |
|
0
day:Group 1 vs. 14day:Group 1 |
-7.960 |
-12.74
to -3.180 |
Yes |
<0.0001 |
|
0
day:Group 1 vs. 14day:Group 2 |
1.440 |
-3.573
to 6.453 |
No |
0.9948 |
|
0
day:Group 1 vs. 14day:Group 3 |
-5.530 |
-10.31
to 0.7503 |
Yes |
0.0129 |

There were significant differences observed in the Rotarod test performance between Group 1 and some of the other groups at certain time points (0 day, 8 day, and 14 day). The mean differences in Rotarod test results between Group 1 and the other groups were statistically significant in two instances, as evidenced by the narrow 95.00% confidence intervals and the adjusted p-values less than 0.05. Specifically, at 14 day, Group 1 had significantly lower performance in the Rotarod test compared to Group 1 and Group 3, as indicated by negative mean differences. Conversely, there were no significant differences in Rotarod test performance between Group 1 and the other groups at 0 day and 8 day, as indicated by the wide confidence intervals that include zero and the adjusted p-values greater than 0.05. In conclusion, this analysis suggests that there were no significant differences in Rotarod test performance between Group 1 and most of the other groups at the initial time points (0 day and 8 day). However, at 14 day, Group 1 exhibited significantly lower performance in the Rotarod test compared to some of the other groups. Shastri (2001)
Table 13
|
Table 13 Showing Statistical Analysis of Radial Arm Test |
||||||
|
Radial Arm |
Group 1 |
Group 2 |
Group 3 |
|||
|
Mean±SEM |
No |
Mean±SEM |
No |
Mean±SEM |
No |
|
|
0 day |
5.83±1.99 |
6 |
5.83±1.14 |
6 |
5.00±1.79 |
6 |
|
8 day |
3.00±1.03 |
6 |
5.80±0.58 |
5 |
3.00±0.58 |
6 |
|
14day |
1.17±0.17 |
6 |
3.60±0.81 |
5 |
1.17±0.17 |
6 |
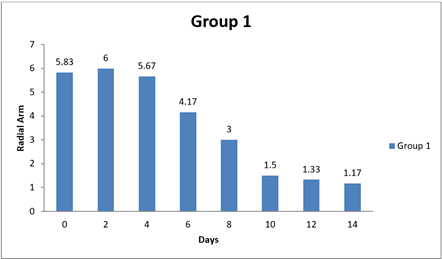
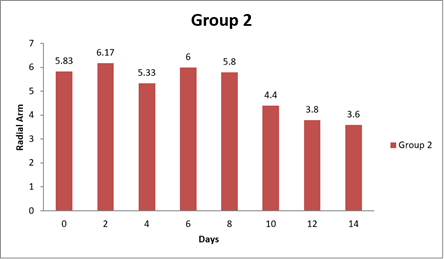
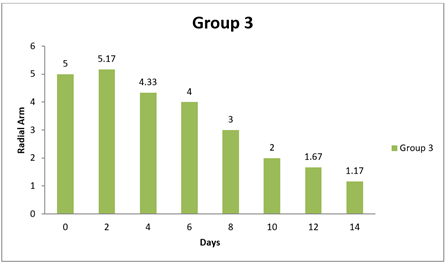
4.5. Result of Radial Arm
Table 14
|
Table 14 Showing Comparison Between Radial Arm Test of all Groups |
||||
|
Dunnett's multiple
comparisons test |
Mean Diff. |
95.00% CI of diff. |
Significant? |
Adjusted P Value |
|
0
day:Group 1 vs. 0 day:Group 2 |
0.000 |
-4.503
to 4.503 |
No |
>0.9999 |
|
0
day:Group 1 vs. 0 day:Group 3 |
0.8300 |
-3.673
to 5.333 |
No |
0.9992 |
|
0
day:Group 1 vs. 8 day:Group 1 |
2.830 |
-1.673
to 7.333 |
No |
0.5137 |
|
0
day:Group 1 vs. 8 day:Group 2 |
0.03000 |
-4.692
to 4.752 |
No |
>0.9999 |
|
0
day:Group 1 vs. 8 day:Group 3 |
2.830 |
-1.673
to 7.333 |
No |
0.5137 |
|
0
day:Group 1 vs. 14day:Group 1 |
4.660 |
0.1575
to 9.163 |
Yes |
0.0376 |
|
0
day:Group 1 vs. 14day:Group 2 |
2.230 |
-2.492
to 6.952 |
No |
0.8620 |
|
0
day:Group 1 vs. 14day:Group 3 |
4.660 |
0.1575
to 9.163 |
Yes |
0.0376 |

There were significant differences observed in the Radial Arm Test performance between Group 1 and one of the other groups at the 14-day time point. Additionally, there was a marginally significant difference at the same time point between Group 1 and another group. However, there were no significant differences in Radial Arm Test performance between Group 1 and the other groups at the initial time points (0 day and 8 day). Specifically, at 14 day, Group 1 showed significantly better performance in the Radial Arm Test compared to Group 1 and Group 3, as indicated by positive mean differences and adjusted p-values less than 0.05. Additionally, there was a marginally significant difference in Radial Arm Test performance between Group 1 and Group 3 at 14 day, as indicated by another positive mean difference and an adjusted p-value of 0.0376. In contrast, at 0 day and 8 day, there were no significant differences in Radial Arm Test performance between Group 1 and the other groups, as indicated by mean differences close to zero and adjusted p-values greater than 0.05. In conclusion, this analysis suggests that Group 1 exhibited better performance in the Radial Arm Test compared to some of the other groups at the 14-day time point, while no significant differences were observed at the earlier time points (0 day and 8 day). Shastri (2011)
Table 15
|
Table 15 Showing Statistical Analysis of Temperature |
||||||
|
Temperature |
Group 1 |
Group 2 |
Group 3 |
|||
|
Mean±SEM |
No |
Mean±SEM |
No |
Mean±SEM |
No |
|
|
0 day |
39.77±0.57 |
6 |
39.47±0.21 |
6 |
39.55±0.58 |
6 |
|
8 day |
39.20±0.27 |
6 |
40.18±0.27 |
5 |
39.60±0.46 |
6 |
|
14day |
39.83±0.21 |
6 |
38.54±1.69 |
5 |
39.14±0.35 |
6 |
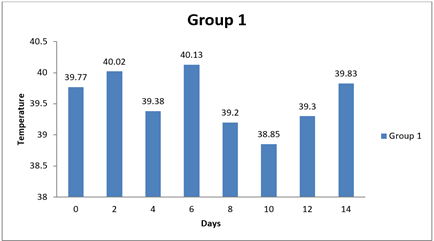
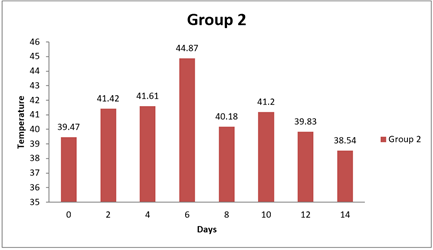
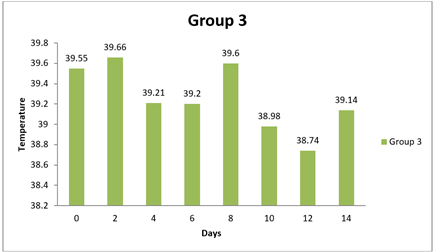
4.6. Result of Temperature
Table 16
|
Table 16 Showing Comparison Between Temperature of all Groups |
||||
|
Dunnett's multiple
comparisons test |
Mean Diff. |
95.00% CI of diff. |
Significant? |
Adjusted P Value |
|
0
day:Group 1 vs. 0 day:Group 2 |
0.3000 |
-3.435
to 4.035 |
No |
0.9997 |
|
0
day:Group 1 vs. 0 day:Group 3 |
0.2200 |
-3.515
to 3.955 |
No |
0.9998 |
|
0
day:Group 1 vs. 8 day:Group 1 |
0.5700 |
-3.165
to 4.305 |
No |
0.9994 |
|
0
day:Group 1 vs. 8 day:Group 2 |
-0.4100 |
-4.327
to 3.507 |
No |
0.9996 |
|
0
day:Group 1 vs. 8 day:Group 3 |
0.1700 |
-3.565
to 3.905 |
No |
0.9998 |
|
0
day:Group 1 vs. 14day:Group 1 |
-0.06000 |
-3.795
to 3.675 |
No |
>0.9999 |
|
0
day:Group 1 vs. 14day:Group 2 |
1.230 |
-2.687
to 5.147 |
No |
0.9940 |
|
0
day:Group 1 vs. 14day:Group 3 |
0.6300 |
-3.105
to 4.365 |
No |
0.9993 |
There were no significant differences observed in the temperature measurements between Group 1 and any of the other groups at all time points (0 day, 8 day, and 14 day). The mean differences in temperature between Group 1 and the other groups were relatively small, and the 95.00% confidence intervals all included zero. Additionally, the adjusted p-values for all comparisons were close to 1, indicating that the observed differences in temperature were not statistically significant. In conclusion, based on this analysis, there were no significant differences in temperature measurements between Group 1 and the other groups during the entire study duration (0 day, 8 day, and 14 day). The temperature values for all groups appeared to be relatively similar and did not vary significantly from each other.
5. Conclusion
Datura, a neurotoxic cerebral deliriant poison, is also classified as "Upavish" in Ayurveda, characterized by its 9 distinct toxic effects. Its active compound, daturine, comprises hyoscine, hyoscyamine, and atropine. This compound functions by blocking acetylcholine receptors, leading to the manifestation of sympathomimetic or parasympatholytic actions, thereby inducing anticholinergic effects. Initial symptoms include a bitter taste, parched mouth and throat, gastric discomfort, dysphagia, cephalalgia, and dysphonia. These are subsequently followed by vertigo, an unsteady gait, impaired muscular coordination, a distinctive flushed facial appearance, xerotic and hyperthermic skin, photophobia, mydriasis, delirium, and somnolence. On occasion, widespread desquamation of the skin may become evident. The cardiac rhythm becomes irregular and sporadic. In instances of lethality, somnolence transitions to stupor, convulsive episodes ensue, eventually leading to coma, culminating in death, primarily due to respiratory insufficiency. In vivo study of Antidotal properties of Sharkara Yukta Gau-Dugdha shows, In Group first all behavioural parameter like Skin and Fur, Eyes, Mucous Membrane, Sleep was normal. Salivation, Lethargy, Coma, Convulsions, Tremors, Diarrhea, Morbidity, Mortality were absent. Changes in weight, food, water intake, rectal temperature, learning memory and muscle strength and motor activity were in normal range. In Group second all behavioural parameter like Skin and Fur, Eyes, Mucous Membrane, Sleep was normal. Salivation, Coma, Tremors were absent. Lethargy, Convulsions, Diarrhea was found in rats and Mortality in one rat was found. Changes in weight was slightly decreased but it was statistically insignificant, food and water intake were slightly decreased but it was statistically significant and rectal temperature was increased in between 2nd to 10th day in toxicity group second but it was statistically insignificant. Response on radial arm was increased, it indicates that showing sample having negative effect on learning and memory and time spent on rota rod was decreased in comparison of group first and third but it was statistically insignificant, it indicates muscle strength and motor activity was decreased in toxicity group second. In Group third all behavioural parameter like Skin and Fur, Eyes, Mucous Membrane, Sleep was normal. Salivation, Lethargy, Coma, Convulsions, Tremors, Diarrhea, Morbidity, Mortality were absent. Changes in weight food water intake, rectal temperature was in normal range, response on radial arm was decreased that showing sample having positive effect on learning and memory and time spent on rota rod was increased in comparison of group 2nd, it indicate muscle strength and motor activity was increased in treatment group third but it was statistically insignificant. The in vivo study suggests that Sharkara Yukta Gau-Dugdha possesses moderate potential in mitigating Datura metel toxicity. The combination of Gau-dugdha and Sharkara might exhibits hepatoprotective, antioxidant properties, soothing effect and acts as a demulcent, thereby reducing the toxic effects of Datura metel alkaloids. Datura metel shares properties similar to visha (poisonous substances), while Gau dugdha (cow's milk) shares properties similar to oja (vital essence or immunity). In the classical texts, Acharya Charak mentioned that the properties of oja are opposite to visha, suggesting that Gau dugdha's properties may counteract the effects of Datura metel due to their opposing nature. According to Dravyaguna Samgreh, Sharkara (sugar) is believed to pacify vata, pitta, and rakta dosha. When it comes to counteract the effects of visha (poison). The probable mode of action of Sharkara could be, it contains madhura rasa, snigdha guna, sheeta virya and madhura vipaka. Sharkara is chaksusya, dhatu vardhaka, hridya, pittahara, vatahara and vrisya may help to balance and soothe the aggravated vishad, vyavayi, vikasi guna which are responsible for symptoms like hyperthermia caused by visha. Datura has deliriant properties and exerts anticholinergic effects. Manifestations of Datura toxicity within toxicity group 2, characterized by lethargy and diminished muscle tone, arise from acetylcholine receptor blockade. Convulsions ensue as neurons become hyperactive, resulting in uncontrolled electrical signaling. In this context, the selected antidote, "sharkara yukta gau-dugdha," appears to mitigate the aforementioned pathophysiological processes by rectifying neuronal dysfunction and counteracting the anticholinergic impact associated with Datura toxicity. Hence from this experimental study entitled “ROLE OF SHARKARA YUKTA GAU-DUGDHA AS PRATIVISHA IN DATURA METEL INDUCED TOXICITY- AN IN VIVO STUDY”, it is clear that the Sharkara yukta gau-dugdha has moderate antidotal effect against Datura induced toxicity. From this study, we can conclude that Sharkara yukta gau-dugdha can reduce the toxic effects of Datura up to some extent. So, In this experimental study null hypothesis (Ho) is rejected and alternative hypothesis (H1) is accepted up to some extent.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Acharya, J.T. (2000). Agnivesha, Revised by Charaka and Dhradabala, Charak Samhita with the Ayurveda – Dipika Commentary of Chakrapanidatta, Chaukhambha Surbharati Prakashan.
Acharya, J.T. (2012). Sushruta; Sushruta Samhita; with the Nibandha Sangraha commentary of Sri Dalhana charya; Reprint Edition, Chaukhambha Surbharati Prakashan, Varanasi.
Paradakara, S. S. (2011). Vagbhata, Ashtanga Hridaya, With the Commentaries Sarvangsundara of Arunadatta and Ayurvedarasayana of Hemadri, (9th Ed.), Chaukhambha Surbharati Prakashan.
Shastri, A. (2011). Sushruta Samhita, Choukhmba Sanskrit Sansthan, Varanasi.
Shastri, H. (2001). Amarasimha; Amarakosha; (4th Ed), Chaukhambha Sanskrit Samsthan, Varanasi.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.