
Evaluation of Oral Toxicological Investigation of a Herbal Composite (Herbodil®) in Experimental Animals
Dr. Soumendra Darbar 1![]()
![]() , Parama Dey 1
, Parama Dey 1![]()
![]() , Srimoyee Saha 2
, Srimoyee Saha 2![]()
![]() , Atiskumar
Chattopadhyay 2
, Atiskumar
Chattopadhyay 2![]()
![]()
1 Principal
Investigator and Scientist, Research and Development Division, Dey’s Medical
Stores (Mfg.) Ltd., 62, Bondel Road, Kolkata-700019, West Bengal, India
2 Scientist,
Research and Development Division, Dey’s Medical Stores (Mfg.) Ltd., 62, Bondel
Road, Kolkata-700019, West Bengal, India
3 Research Scientist, Faculty Council of Science, Jadavpur University,
Kolkata-700032, West Bengal, India
4 Former principal Secretary, Faculty Council of Science, Jadavpur
University, Kolkata-700032, West Bengal, India
|
|
ABSTRACT |
||
|
Purpose: The aim of the
investigation was to find out the degree of toxicity of the herbal composite
for humans, animals, or the environment. Acute and 28 days repeated sub-acute
oral toxicity study of herbal composite (Herbodil®)
carried out as per the current OECD guidelines. Materials
and Methods: 2000mg/kg of the herbal composite was orally administered to the
animals to find out the acute toxicity. The treated animals were observed for
toxic signs at thirty min, one, two and four hours and thereafter once a day
for the next 14 days. In sub-acute study i.e.,28 days repeated dose oral
study, the mice were segregated into four groups (two set for each sex) of
six mice each. Group-1 mice served as a control (untreated). Group II mice
consumed lower dose of herbal composite i.e., 100 mg/kg, Group III mice
consumed middle/moderate dose i.e., 200 mg/kg and Group IV mice received high
dose of 400 mg/kg (orally) once daily for 28 days respectively. Results: This experimental in
vivo study confirm that neither the acute toxicity study of herbal composite
at the dose level of 2000mg/kg nor the sub-acute 28 days oral toxicity study
developed any toxic signs, behavioural changes, or
mortality during the whole study. Haematological
and biochemical parameters dose not changes during
the sub-acute study. Relative body weight of the mice also not
change after the study. Conclusion: Experimental results
obtained from the current investigation suggest that LD50 of developed herbal
composite was >2000 mg /kg and the herbal composite is completely safe and
non-toxic for therapy. |
|||
|
Received 27 January 2023 Accepted 06 May 2023 Published 29 May 2023 Corresponding Author Dr.
Soumendra Darbar, dr.soumendradarbar@deysmedical.com DOI 10.29121/jahim.v3.i1.2023.27 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Acute Oral Toxicity, 28-Day Sub Acute
Toxicity, Herbal Fomulation, Haematology,
Liver Function Test |
|||
1. INTRODUCTION
In recent years herbal medicine is most popular and effective medication throughout the globe. These drug mainly composed of various medicinal plants and very much effective for curing disease Ramdas et al. (2020), Rickert et al. (1999). Experimental study showed that the herbal combination have enormous medicinal effects and very limited deleterious/adverse effects in relative to individual plant ingredient Asira et al. (2014). According to the report of World Health Organization (WHO) it is recommended that approximately 79 to 82 % population all over the glove think on these “therapeutic alternative remedies” are derived from plant. Now both developing and developed countries where tremendously depends on herbal medication as effective and safe medication Darbar et al. (2010), Darbar et al. (2009), Darbar et al. (2000). Last few years use of herbal medicines and its formulations are extensively used for different diseases and health related problems. In this context researchers are continuously engaged to developed plant derived product those are medically beneficial. Medical practitioners are frequently prescribed Herbal formulation and natural medicines in developed countries for the treatment of numerous illnesses Aneela et al. (2011).
Toxicological investigation is the important issue in pharmacology that deals with the adverse and harmful effect of bio active substance on living mammalian system prior to the use as drugs, medicine or chemical in clinical use Mir et al. (2013), Darbar and Chattopadhyay (2018). In 21st century toxicity study of chemicals, food substances, pharmaceuticals, etc. have the significant interest to overcome the unwanted effects after application of medicine Asira et al. (2014). After the clinical toxicity study the collected data are very useful to draw the interpretation about the drug and its safety profile. That’s why Biosafety level of the plant product is very crucial in drug discovery Darbar et al. (2018). According to OECD guidelines, acute toxicity is the preliminary investigation and assessment of the health-related complications of a test substance in one or more doses during a single period. So, result of the test substance deliver the particulars on possible health hazards even the exposure is too short.
Various plant parts those are extensively used for preparation of natural medicine have sometimes showed toxicity when used either acutely or sub-chronically Darbar and Chattopadhyay (2018), Asira et al. (2014). Due to the lack of experimental evidences plant based products sometimes showed toxic effect upon human and animals and raised question about the safety and efficacy of the product Darbar et al. (2021), Darbar et al. (2019). Common routine toxicological investigation in pre-clinical study proved that the medicine is safe in human use, and it does not show adverse effects upon mammalian system.
Our developed herbal formulation (Herbodil®) mainly composed of Indian medicinal plans namely Ocimum sanctum, Piper nigrum, Piper longum and Adhatoda vasica are very useful medication in common cough and cold. Previous studies of the individual ingredients showed that all the herbs used in this medicine had no toxic effects on animals. But in combination the toxicity is not carried out still date. So, our aim and objective of this study is to determine the safety of the developed natural product by single dose oral acute toxicity (14 days) and sub-acute (28days) repeated doses oral toxicity in experimental mice model. Organization for economic cooperation and development’s (OECD) guidelines (423 & 407) were strictly followed to conduct the experiment.
2. METHODOLOGY
2.1. HERBAL INGREDIENTS
In this study the herbal formulation contains four Indian
Medicinal plants namely Ocimum sanctum, Piper nigrum, Piper longum and Adhatoda vasica. All the plants were procured
from registered suppliers and authenticated by a renowned Botanist.
2.2. PREPARATION OF POLYHERBAL FORMULATION
The plants of Ocimum sanctum, Piper nigrum, Piper longum and Adhatoda vasica were collected from the registered vendor. All the plants and plants parts were thoroughly cleaned with plain and distilled water and then dried under sunlight. The dried plants were send to product development section for preparation of powder. Dry powder was collected and then followed the sieving process by the use of sieve No.40. Powder was stored for the extraction process. 500 gm of powdered plant materials were weighted and packed in the soxhlet extraction assembly and extracted by using solvents of increasing polarity by continuous hot extraction, for 48 Hrs. The aqueous extraction was carried out by cold-maceration method.
2.3. STABILITY STUDIES PROFILE OF THE DEVELOPED FORMULATION
Stability profile of the developed herbal drug was investigated according to WHO guideline with proper environmental condition.
2.4. EXPERIMENTAL ANIMALS
This study was carried out upon healthy adult and disease-free Mice (both male and female) weighing 25-28 g. The experimental animals were collected from the CPCSEA registered central animal house. Mice were kept and housed in clean and dry polypropylene cages, placed in environmentally monitored room with a 12 h light 12 h dark cycles. Standard pellet diet and pathogen free drinking water (ad libitum) were given to the animals except when fasting was required during the study. The research protocol and procedure were carried out in accordance with IAEC rules and CPCSEA regulation (Dey’s/P/IAEC/ 04/16-17).
2.5. ASSESSMENT OF ACUTE TOXICITY STUDY
According to the protocol of organization for economic cooperation and development (OECD) revised fixed dose procedure for acute toxicity testing (OECD guideline 420, 2001) fourteen days acute toxicity study was performed. Thirty Swiss albino Mice (both sex) were divided into six groups. Herbodil extract at a dose of 2000 and 5000 mg/kg were administered to the animals (Figure 6) for assess the acute toxicity. After application of drug the changes in fur and skin, eyes sight changes, secretion of mucus membranes, diarrhoea, salivation, changes of normal sleep pattern, behaviour pattern, coma, mortality, tremors, moribund, ill health or any visible reaction to treatment were critically observed for the first hour, then hourly for next 3 hrs and finally periodically until 48hrs. Clinical observation was carried out upto 14 days with closely monitoring Chitme et al. (2004).
2.6. 28 DAY SUB-ACUTE TOXICITY STUDY
In this study 28 day oral toxicity study (sub-acute) was conducted as per OECD guidelines OECD-407. Swiss albino mice were taken and segregated into 4 groups of 12 mice each (6 males mice and 6 females mice). The initial body weights were taken by using a digital weighing balance and the data were recorded. Normal control mice (group I) received only distilled water, while groups II–IV received herbal composite (Herbodil®) at the dose of 100, 200 and 400 mg/kg respectively (Figure 6). Experimental sample were given to the animals through oral route once a day for 28 days. Abnormalities and sings during the study period was noted and recorded. Body weights of mice were recorded as regular basis. On 28th day of treatment, animals were placed in individual metabolic cages for 24hr. On the 29th day the fasting mice were sacrificed with proper euthanasia. Blood was taken from the retro orbital plexus with and without anticoagulant (ethylene diamine tetra acetate - EDTA), using capillary tubes for biochemical and hematological analysis.
2.7. HAEMATOLOGICAL STUDY
After the last treatment (28th day) of tested drug the entire mice were kept for overnight fasting (water ad libitum). On 29th day animals those were fasted anaesthetized using isoflourane with standard dose and blood samples were taken for hematological studies by using heparinized tubes. Blood smears were detected by blood-cell count (Sysmax-K1000 Cell Counter). Total red blood cell (RBC), haemoglobin (Hb), platelets, total white blood cell (WBC), haematocrit (HCT) were measured, and data were preserved for preparation of report.
2.8. LIVER FUNCTION TEST
Various biochemical parameters like total cholesterol, Aspartate Transaminase (AST), Alanine Transaminase (ALT) and Alkaline Phosphatase (ALP) were measured by using different biochemical kits (Autospan LiquidGold, Span Diagnostics Ltd., India). The tests were performed by the protocols described by the corresponding manufacturers. Semi-automated biochemical analyser were used for measure the sample.
2.9. STATISTICAL ANALYSIS
Experimental results were expressed as mean ± SEM. P value < 0.05 was considered statistically significant. Collected data obtained from the toxicity studies were analysed by Student’s t-test using Graph Pad prism 5.0 to determine significant difference between the means of control and test groups.
3. RESULTS AND DISCUSSION
In recent years plants-based formulation has maintained greater interest all over the world and its use is extensively increasing. According to WHO report 78-82 % global population mainly used natural medicines for curing diseases. For safe and symptomatic medication toxicity study is mandatory to fulfil the regulatory compliances Ayyanar and Subash (2012), Darbar et al. (2022)
Systemic toxicological investigation and adverse effects both
single and multiple doses of a selective substance was evaluated through acute
systemic toxicity as per OECD guideline. This toxicity study upon animals was
universally used for detection of probable hazards of chemicals on humans.
Mortality is the major endpoint of the study. Detection of tolerated dose and
detected Dhankhar et al. (2011). In the present
study medicinal plants such as Ocimum sanctum, Piper nigrum, Piper longum and Adhatoda vasica were used for preparation of
formulation (Table 1). Preliminary study stated
that the formulation was stable for 36 months.
Table 1
|
Table 1 Details
Composition of Developed Herbal Composite (Herbodil(R)) Each 5mL contains: Aqueous extract of: |
|||||
|
S. No. |
Scientific Name |
Common Name |
Family |
Quantity |
Parts Used |
|
1. |
Ocimum Sanctum |
Tulasi |
100 mg |
All Parts |
|
|
2. |
Piper
nigrum |
Morich |
Piperaceae |
25 mg |
Flower |
|
3. |
Piper
longum |
Pippali |
Poaceae |
25 mg |
Root |
|
4. |
Adhatoda Vasica |
Vasaka |
|
100 mg |
Leaves |
List of toxic indicators such as alteration of normal central and autonomic nervous systems function, function of cardiovascular system, stress, eye irritation, convulsion, muscular spasms, presence of tremors, stereotypic behaviour, sedation, lungs behaviour & respiratory distress, pilo erection, muscular grip etc. were detected. Apart from this some others external observation such as response to sensory stimuli, limb paralysis, sleep, salivation, lethargy, diarrhoea, coma, and mortality were observed on daily basis and recorded the data with special attention (Table 2). The observed results indicate that no any death or signs of toxicity in the treated animals (Table 3). Details changes in the gross body weight of both sex mice (male and female mice) were recorded and are compared with control group (Table 4). Further, there were no any gross pathological abnormalities, which established that the LD50 was found to be greater or above than 2000 mg/ kg b.wt. which is very high as recommended daily normal doses.
Table 2
|
Table 2 Clinical Symptoms Observations During the Toxicity
Study of Mice at 2,000 mg/kg Dose of Herbal Formulation (Herbodil®) |
|||
|
Signs and symptoms |
Clinical Observations (Day) |
||
|
|
Day 1 |
Day 7 |
Day 14 |
|
Activity of Somatomotor |
Usual |
Usual |
Usual |
|
Behavior |
Usual |
Usual |
Usual |
|
Condition of mucous and eye membranes |
Usual |
Usual |
Usual |
|
Status of Salivation |
Not detected |
Not detected |
Not detected |
|
Condition of Fur and Skin |
No change |
No change |
No change |
|
Symptoms of Diarrhoea |
Not detected |
Not detected |
Not detected |
|
Tremors/ convulsions |
Not detected |
Not detected |
Not detected |
|
Mortality (Death) |
Nil |
Nil |
Nil |
|
Other condition/symptoms |
Nil |
Nil |
Nil |
Table 3
|
Table 3 Acute Toxicity Study Herbal Formulation (Herbodil®) |
|||
|
Groups |
Dose |
D/T |
Mortality |
|
Control |
- |
Nil |
Nil |
|
PHF treated |
2000 mg/kg. |
None |
None |
|
D/T: Dead/Treated mice. None: Symptoms of toxic sign were not
detected throughout the observation period. |
|||
In sub-acute toxicity study of herbal composite didn’t produce any clinical signs of toxicity. No death recorded during the study period. Changes of gross body weight were not detected in the study. Water intake and consumption of food of different treatment groups were observed and found to be insignificant when compared to the normal untreated groups. In the group IV (200mg/kg) significant rise (P < 0.05) in the body weight of mice was noticed, while no such significant difference was detected in all other groups (Table 4). The finding suggests that, during the toxicity study the herbal composite has no hazardous or toxic substances and safe for intake.
Table 4
|
Table 4 End Result
of PHF (Herbodil®) on Relative Body Weight
of Mice |
||||
|
Gross Body wt. (gm) |
Control |
Polyherbal Formulation (Herbodil®)
mg/kg |
||
|
|
|
100 |
200 |
400 |
|
Initial weight |
26.1±0.37 |
25.9±0.29 |
25.8±0.31 |
26.0±0.28 |
|
Final weight |
35.4±0.41 |
34.8±0.33 |
35.1±0.36 |
36.8±0.36 |
|
Body weight gain or loss |
9.3(+) |
8.9(+) |
9.3(+) |
10.8(+) |
Haematological values showed that Haemoglobin (Hb), Packed Cell Volume (PCV), Red Blood Cells (RBC), White Blood Cell (WBC), Platelets, Neutrophils, Eosinophil’s, Basophils values have no such alteration in comparison to normal animals. Monocytes and Lymphocytes count were within the normal prescribed physiological limits for rodents and insignificant when compared with the normal untreated groups (Table 6). Hence, it is suggested that there was no any serious toxicological implication.
Table 5
|
Table 5 End Result Herbal Formulation (Herbodil®)
on Relative Organ Weight of Mice |
||||
|
Organ wt. (gm) |
Control |
Polyherbal Formulation (Herbodil®)
mg/kg |
||
|
|
|
100 |
200 |
400 |
|
Lung |
0.214±0.05 |
0.204±0.09 |
0.198±0.07 |
0.211±0.08 |
|
Heart |
0.156±0.08 |
0.144±0.03 |
0.151±0.06 |
0.149±0.06 |
|
Spleen |
0.088±0.004 |
0.077±0.002 |
0.081±0.005 |
0.092±0.006 |
|
Liver |
1.246±0.8 |
1.172±0.12 |
1.198±0.09 |
1.184±0.11 |
|
Kidney |
0.268±0.08 |
0.255±0.04 |
0.247±0.04 |
0.278±0.09 |
Table 6
|
Table 6 End
Result of Herbal Formulation (Herbodil®)
on Haematological Values of Mic |
||||
|
Haematological
Parameters |
Control |
Polyherbal Formulation (Herbodil®)
mg/kg |
||
|
|
|
100 |
200 |
400 |
|
Haemoglobin(gm/dl) |
10.24±0.07 |
10.11±0.05 |
10.43±0.07 |
10.97±0.09 |
|
Haematocrit
(PCV) (%) |
31.28±0.12 |
28.91±0.11 |
34.51±0.09 |
32.74±0.18 |
|
RBC (×106
uL−1) |
5.99±0.04 |
5.76±0.07 |
6.18±0.05 |
6.04±0.08 |
|
Platelets
(×103 uL-1) |
345.01±3.41 |
366.0±2.88 |
351.9±4.08 |
366.4±3.56 |
|
WBC (×103 uL-1) |
8.34±0.04 |
7.98±0.06 |
9.34±0.08 |
10.12±0.07 |
|
Monocytes
(%) |
2.56±0.08 |
2.88±0.04 |
3.67±0.04 |
3.89±0.09 |
|
Lymphocytes
(%) |
44.56±1.87 |
48.71±2.09 |
45.28±1.64 |
52.98±1.68 |
|
Neutrophils
(%) |
19.11±0.45 |
21.21±0.32 |
18.07±0.41 |
19.56±0.37 |
|
Eosinophils
(%) |
2.27±0.004 |
2.34 ±0.005 |
2.44±0.002 |
2.45±0.007 |
|
Basophils
(%) |
0.41±0.008 |
0.38±0.004 |
0.44±0.006 |
0.47±0.003 |
Aspartate amino transaminase (AST) and Alanine amino Transaminase (ALT) are the main two liver function detection enzymes are commonly used in the assess of liver damage and liver related complications by various toxins and drugs (Figure 1 & Figure 2). So, to establish the toxic hazards during hepatic metabolism of drug, level of transaminase activity plays a vital role. The main two liver function enzymes AST and ALT which is the index of hepatocellular damage or injury didn’t show any significant changes in the polyherbal formulation treated groups. In the various part of the body another enzyme alkaline phosphatase (ALP) is essential for liver metabolism. This enzyme is profoundly found in the intestine, bones, and liver. Determination of ALP is commonly used to diagnose liver damage or bone disorders Darbar et al. (2021), Darbar et al. (2020). In the present study content of alkaline phosphatase (ALP) not significantly altered in compared with control (Figure 3). Lipid parameters Total Cholesterol didn’t show any significant changes when compared with the control groups (Figure 4).
Figure 1
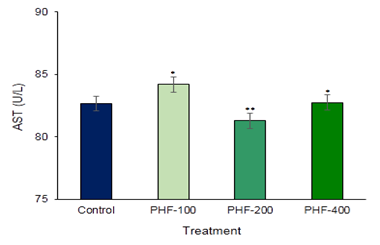
|
Figure 1 Toxicological Investigation of Developed Herbal Formulation (Herbodil®) on Serum Aspartate Amino Transaminase (AST) Activity. Values Expressed are Mean ± SE (n=10). *Significantly different with Normal Group P < 0.05 and **Significantly different with Normal Group P < 0.001 |
Figure 2
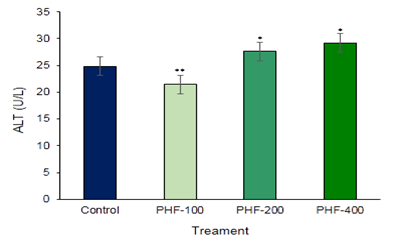
|
Figure 2 Toxicological Investigation of Developed Herbal Formulation (Herbodil®) on Serum Alanine Amino Transaminase (ALT) Activity. Values Expressed are Mean ± SE (n=10). *Significantly different with Normal Group P < 0.05 and **Significantly different with Normal Group P < 0.001 |
Figure 3
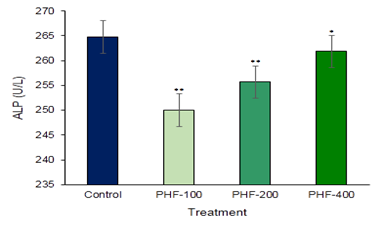
|
Figure 3 Toxicological Investigation of Developed Herbal Formulation (Herbodil®) on Serum Alkaline Phosphatase (ALP) Activity. Values Expressed are Mean ± SE (n=10). *Significantly different with Normal Group P < 0.05 and **Significantly different with Normal Group P < 0.001 |
Figure 4
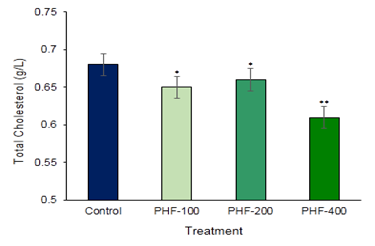
|
Figure 4 Toxicological Investigation of Developed Herbal Formulation (Herbodil®) on Serum Total Cholesterol Activity. Values Expressed are Mean ± SE (n=10). *Significantly different with Normal Group P < 0.05 and **Significantly different with Normal Group P < 0.001. |
Figure 5

|
Figure 5 List of Active Ingredients Present in the Developed Herbal Formulation-Herbodil® |
Figure 6
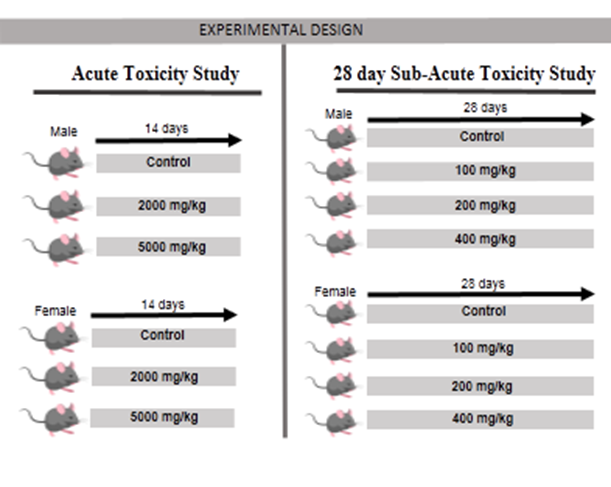
|
Figure 6 Experimental Protocol |
Our prepared plant-based combination is developed by mixing four popularly used Indian medicinal plants in a doses content (Figure 5). Results from our above experiment clearly stated that this developed combination was completely nontoxic and did not produce organ related toxicity at all dose levels.
4. CONCLUSION
In this study Acute and 28-day repeated toxicity results
predict that LD50 of multi herbal composite (HC) was >2000mg/kg.
The formula is safe and nontoxic upon mammalian system. Cost of the formulation
is within the capacity of common people. In future long term toxicity study
both rodent and non-rodent as well as clinical trials may be a strong weapon to
establish the safety and efficacy of the drug.
AUTHORS’ CONTRIBUTION
Soumendra Darbar and Atiskumar Chattapadhyay conceived and designed the experiment. Soumendra Darbar and Srimoyee Saha conducted the animal and biochemical experiments. Soumendra Darbar and Parama Dey wrote and revised the manuscript.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors are thankful to Prof. S K Pal, Senior Professor, Department of Chemical, Biological & Macromolecular Sciences, S N Bose National Centre for Basic Sciences, JD Block, Sector III Salt Lake City for his guidance and valuable suggestion during this investigation. The authors also convey their heartiest gratitude to Mr. Gautam Dey, M.D. & Mr. Ranajit Dey, Jt. M.D. for facilities and encouragement during this investigation.
REFERENCES
Aneela, S. De Somnath, K.K. Lakshmi, N.S.K. Choudhury, S.L. Das, K.V. (2011). Sagar, Acute Oral Toxicity Studies of Pongamia Pinnata and Annona Squamosa on Albino Wister Rats. International Journal of Research in Pharmacy and Chemistry, 1(4), 820-824.
Asira, T. Shariq, S. Roohi, Z. (2014). Acute Toxicity Study of a Polyherbal Unani Formulation Habbe Shifa in Experimental Animal Model. Indian Journal of Traditional Knowledge, 13(1), 171-174.
Ayyanar, M. Subash, B.P. (2012). Syzygium Cumini (L.) Skeels : A Review of its Phytochemical Constituents and Traditional Uses. Asian Pac J Trop Biomed, 2(3), 240-246. https://doi.org/10.1016/S2221-1691(12)60050-1.
Chitme, H.R. Ramesh, C. Sadhna, K. (2004). Study of Antidiarrhoeal Activity of Calatropsis Gigantean Inexp Erimental Animals. J Pharmacol Pharm Sci, 7(1), 70-75.
Darbar, S. Bhattacharya, A. Chakraborty, M.R. Chattopadhyay, S.P. (2010). Livina, A Polyherbal Preparation Protects Liver Against Aceclofenac-Induced Hepatic Insult in Sprague-Dawley Rats : A Comparison with Silymarin. Pharmacology online, 2, 889-907.
Darbar, S. Chattopadhyay, S.P. (2018). Single Dose Acute Oral Toxicity of Livina, a Polyherbal Formulation in Mice Model. European Journal of Pharmaceutical and Medical Research, 5(2), 492-495.
Darbar, S. D., Saha, S., Chattopadhyay, S., & Chattapadhyay, A. (2020). Anti-Stress Activity (in-vivo) of Multi Herbal Capsule-Trasina® in Experimental Murine Model. Asian Journal of Pharmaceutical Research and Development, 8(5), 52-58. https://doi.org/https://doi.org/10.22270/ajprd.v8i5.839.
Darbar, S. Saha, S. Pramanik, K. Chattopadhyay, A. (2000). Preliminary Assessment of Acute and 28-Day Repeated Dose Oral Toxicity of a Newly Developed Herbal Mixture on Experimental Animal. Indian Journal of Pharmaceutical education and Research, 54(1),135-142. https://doi.org/10.5530/ijper.54.1.16.
Darbar, S. Saha, S. Pramanik, K. Chattopadhyay, A. (2018). Preliminary Acute Oral Toxicity Study of a Newly Developed Herbal Formulation. World J Pharm Res, 7(5), 924-930.
Darbar, S. Saha, S. Pramanik, K. Chattopadhyay, A. (2019). Toxicological Assessment of Silver Nanoparticles Synthesized through Green Route using Andrographis paniculata. Journal of Nanoscience and Technology, 5(1), 619-621. https://doi.org/10.30799/jnst.175.19050111.
Darbar, S. Saha, S. Pramanik, K. Chattopadhyay, A. (2021). Antioxidant and Immunomodulatory Effect of Akss16-Liv01-A Multi Herbal Formulation Against Ethanol Induced Liver Dysfunction in Mice. Clinical Phytoscience, 7(1),1-20. https://doi.org/10.1186/s40816-021-00312-1.
Darbar, S. Saha, S. Pramanik, K. Chattopadhyay, A. (2022). Haematological Modulations by Fixed Dose Combination (FDC) of Tramadol Hydrochloride/Paracetamol (THP). Frontiers in Clinical Drug Research-Hematology, 5, 154. https://doi.org/10.2174/9789815039535122050007.
Darbar, S., Chakraborty, M. R., Chattarjee, S., & Ghosh, B. (2009). Protective Effect of Livina, A Polyherbal Liquid Formulation Against Ethanol Induced Liver Damage in Rats. Ancient science of life, 28(3), 14–17.
Darbar, S., Saha, S., & Chatopadhyay, A. (2021). Ethanol Intoxicated Hepatic Oxidative Stress Mitigated by Poly-Herbal Formulation – Trasina® in Murine Model. Innoriginal : International Journal of Sciences, 8(3).
Dhankhar, S. Ruhil, S. Balhara, M. Dhankhar, S. Chhillar, A. (2011). Aegle Marmelos (Linn.) Correa : A Potential Source of Phytomedicine. J Med Plant Res, 5(9), 1497-1507.
Mir, A.H. Sexena, M. Malla, M. Y. (2013). An Acute Oral Toxicity Study of Methanolic Extract from Tridex Procumbens in Sprague Dawley's Rats as Per Oecd Guidelines. An Acute Oral Toxicity Study of Methanolic Extract from Tridex Procumbens in Sprague Dawley's Rats as Per Oecd Guidelines, 3(1), 16- 20.
Ramdas, B. P. Santosh, N. B. Sangameswaran, B. Popat, B. M. Shantaram, G. K. Vinayak, K. D. (2020). Assessment of Acute and 28-Day Sub-Acute Oral Toxicity of a Polyherbal Formulation in Rats. Int J Pharmacogn Chinese Med, 4(1), 1-7. https://doi.org/10.23880/ipcm-16000200.
Rickert, K., Martinez, R. R., & Martinez, T. T. (1999). Pharmacist Knowledge of Common Herbal Preparations. Proceedings of the Western Pharmacology Society, 42, 1–2.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.