
ORAL HEALTH PROTECTION THROUGH AN AYURVEDA POLY HERBAL FORMULATION- AN IN VITRO STUDY
Saranya Nadesakanthan 1![]()
![]() , Asanka Sanjeewa 1
, Asanka Sanjeewa 1![]()
![]() , Inoka
Uluwaduge 2
, Inoka
Uluwaduge 2![]()
![]() , Priyani
Peiris 3
, Priyani
Peiris 3![]()
![]() , Fahara
Meedin 4
, Fahara
Meedin 4![]()
![]() , Samantha Bandara 5
, Samantha Bandara 5![]()
![]()
1 Graduate, B. Sc. Honours, Medical
Laboratory Sciences, Faculty of Allied Health Sciences, University of Sri
Jayewardenepura, Sri Lanka
2 Professor, Department of Basic Sciences, Faculty of Allied Health Sciences, University of Sri Jayewardenepura, Sri Lanka
3 Professor, Department of Shalya – Shalakya, Faculty of Indigenous Medicine, Gampaha Wickramarachchi University of Indigenous Medicine, Yakkala, Sri Lanka
4 Senior Staff Technical Officer, Department of Medical Laboratory Sciences, Faculty of Allied Health Sciences, University of Sri Jayewardenepura, Sri Lanka
5 Ph.D. Student Centre of Excellence for Alzheimer's Disease Research and Care, School of Medical and Health Sciences, Edith Cowan University Level 2, Ralph, and Patricia Sarich Neuroscience Research Institute (SNRI)8 Verdun St, Nedlands Western Australia
|
|
ABSTRACT |
||
|
Introduction: Natural medicines prescribed by the traditional Ayurvedic medical system of Sri Lanka have been extensively used to treat and prevent oral diseases. Objective: Here, we investigated the antimicrobial effect of Ayurveda polyherbal formulation, mentioned in authentic text as Gandusha (mouth wash) consisting of Jasminum officinale leaves, Terminalia chebula fruits, Tinospora cordifolia stem, Desmodium triflorum whole plant and Glycyrrhiza glabra roots. Methods: Anti-microbial susceptibility of the poly herbal preparation against common oral pathogens (Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis) was tested using agar well diffusion method. Minimum inhibitory (MIC) was expressed in mg of freeze-dried extract per millilitres of agar solution. Results: The plant extract has considerable antimicrobial activity against Staphylococcus aureus and Enterococcus faecalis. The lowest MIC of 250mg/ml was shown against Enterococcus faecalis and Staphylococcus aureus. Conclusions: The polyherbal formulation is effective in mitigating the
bacterial growth and hence enhance the oral health protection. |
|||
|
Received 29 December 2022 Accepted 23 January 2023 Published 08 February 2023 Corresponding Author Inoka Uluwaduge, deepthiuluwaduge@sjp.ac.lk DOI 10.29121/jahim.v3.i1.2023.26 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Oral Health, Poly Herbal Formulation,
Plant Extract |
|||
1. INTRODUCTION
In Susruta Samhita, there are numerous descriptions on periodontal diseases, oral mucosal diseases, and dental caries. Singhal (1972) Among oral diseases, periodontal disease and dental caries are major oral health hazards WHO Technical Report Series. (1995). Dental plaque is the main etiological factor for periodontal disease and dental caries William et al. (2001). Therefore, since ancient time Ayurveda and Sri Lankan traditional medical system has paid a significant attention on preventive and curative aspects of oral diseases. Further, Charaka Samhita has emphasized on modalities of tooth brushing, and oral cleansing through mouth rinses to maintenance of oral hygiene in Dinacharya (daily personal hygienic measures) Agnivesha (2008). Therefore, Ayurveda medical science recommends special local therapeutic measures such as Gandusha (Mouth wash), Kavala (Gargles) and Pratisarana (local application) to prevent dental plaque formation Sharangadhara. (2013).
Anti-bacterial behaviour of the medicinal plants is attributed to potential bioactive compounds that are meant to reduce the bacterial load in the oral cavity thus preventing plaque formation Kadam et al. (2011).
This research is aimed at investigating antimicrobial effect of an Ayurveda polyherbal formulation mentioned in authentic text as a Gandusha (mouthwash). The ingredients of this poly herbal formulation are leaves of Jasminum officinale, fruits of Terminalia chebula, stem of Tinospora cordifolia, whole plant of Desmodium triflorum and roots of Glycyrrhiza glabra. This poly herbal preparation is long been used in Sri Lankan Ayurveda as an effective oral treatment. However, none of the research studies have been carried out to prove its´ clinical effectiveness on scientific basis. Therefore, this study was aimed to fill the gap of knowledge from Ayurveda presumption to modern scientific evidence. Further, Ayurveda Materia medica have been proven their safety and efficacy through several thousand years of use.
2. METHODS
2.1. STUDY DESIGN AND SETTING
This is a laboratory
based In-vitro experimental study carried out at the microbiology laboratory, Department
of Medical Laboratory Sciences, Faculty of Allied Health Sciences, University
of Sri Jayewardenepura, Sri Lanka. All the plant materials were identified and
authenticated by the Department of Dravyaguna, Institute of Indigenous Medicine, University of
Colombo, Sri Lanka.
2.2. PREPARATION OF AQUEOUS EXTRACT
Aqueous extract was prepared using dried pericarp of T. chebula, dried stem of T. cordifolia,
roots of G.
glabra, 75 g each, tender leaves of J.
officinale and whole plant of D. triflorum, 150 g of each. The
ingredients were boiled in a clay pot under low heat with 12 cups of water
(2880 ml) to reduce the volume up to 2 cups (480 ml) according to Ayurveda Gandusha
preparation method Sharangadhara. (2013). The polyhedral extract was filtered,
and filtrate was used for the assessment of in-vitro antibacterial efficacy. Figure 1
Figure 1
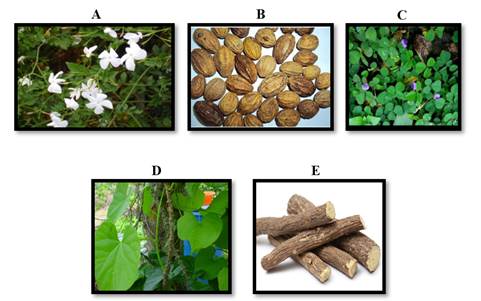
|
Figure 1 Ingredients of Poly
Herbal Preparation A. J. officinale B. T. chebula C.
D. triflorum D. T. cordifolia E. G. glabra |
2.3. PREPARATION OF POSITIVE CONTROLS
(ANTIBIOTIC SOLUTIONS) FOR ABST
1) Amoxicillin – 10 mg was dissolved in sterile distilled water (5 ml). Concentration of amoxicillin was 2 mg/ml.
2) Penicillin –Commercially available and concentration was 10000 units/ml.
3) Gentamicin –Commercially available as liquid and concentration was 40mg/ml.
2.4. PREPARATION OF BACTERIAL CULTURE MEDIA
Mueller Hinton Agar Media (MHA) - Mueller Hinton
agar powder (26.6 g) and bacteriological agar powder (2.1 g) was dissolved
completely in 700 ml of distilled water Kanchan et al. (2019).
The mouth of the conical flask was wrapped using aluminium foil and was sterilized
by autoclaving and allowed to cool at room temperature. Cooled MHA medium was poured
into sterilized disposable culture plates and allowed to solidify at room
temperature.
Molten Agar –Agar
(0.4 g) was dissolved with 20ml distilled water, autoclaved at 120º C for 15 minutes
and kept in a water bath at 60ºC.
2.5. MICROORGANISMS
AND CONTROLS
Bacterial
species representative of key pathogens causing oral diseases and widely
accepted as testing strains for antimicrobial activity were chosen. Two
pathogenic Gram-positive bacteria, Staphylococcus aureus (ATCC 25923)
and Enterococcus faecalis (ATCC 29212); two pathogenic Gram-negative
bacteria, Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC
27853) were used.
All microbial cultures were collected from Quality Control Laboratory, Department of Microbiology, Medical Research Institute, Colombo, Sri Lanka and the Department of Microbiology, National Hospital, Colombo, Sri Lanka.
2.6. STANDARD MICROBIAL CULTURE PREPARATIONS
Staphylococcus aureus (ATCC 25923), Escherichia
coli (ATCC 25922), Pseudomonas
aeruginosa (ATCC 27853) and Enterococcus
faecalis (ATCC 29212) were subcultured by
using freshly prepared MHA plates separately to obtain 24-hour fresh cultures
for the antimicrobial assessment. They were incubated overnight at 370C.
At the end of the incubation period, small colonies were taken from sterilized
wire loop and dissolved in normal saline (0.9%). The bacterial suspension was adjusted
by adding sterilized normal saline until the turbidity matched with of 0.5
McFarland standard Khan et al. (2013). Four bacterial
suspensions were prepared separately from each culture collection and labelled
accordingly.
2.7. DETERMINATION OF ANTI-MICROBIAL ACTIVITY BY AGAR WELL DIFFUSION METHOD
The study was
based on anti-microbial susceptibility test by using ATCC cultures as “standard
microorganisms”, sterile normal saline as a “negative control” and antibiotic
solutions as “positive controls” using agar well diffusion method.
The inoculum was smeared over the MHA plate using a sterile
cotton swab in three directions, rotating the plate approximately 600 to
ensure uniform microbial growth. Five wells were made in the agar surface. The
wells were made with 8mm in diameter and 4mm in depth. The bottom of the wells
was sealed by adding a drop of molten agar into each using sterile plastic
Pasteur pipette. All the procedures were carried out aseptically. The wells contained.
1) Aqueous extract (75µl) as the test sample
2) Amoxicillin solution (75µl) of and penicillin solution (75µl) as positive controls against gram positive microorganisms (S. aureus and E. faecalis).
3) Sterile distilled water (75µl) as the negative control.
4) Gentamicin (75µl) antibacterial preparation as positive control against gram negative microorganisms (E. coli and P. aeruginosa).
The plates were labelled systematically for better identification before incubation.
The plates were
wrapped with para films carefully without disturbing the solutions in the wells
and kept for 15 minutes to diffuse the antibacterial solutions, the plant
extract and the sterile distilled water to the media and then incubated at 370C
for 24 hours. At the end of the incubation period, diameter of inhibition
zones was measured for the plant extract, positive control, and negative
control for each microorganism. In agar well diffusion method, any zone of inhibition
observed was considered as significant. The
well diffusion assay was done in triplicates for each isolate and average
inhibition zone was calculated Peiris et al. (2019).
2.8. DETERMINATION OF MINIMUM INHIBITORY CONCENTRATION (MIC) OF THE POLY
HERBAL PREPARATION
The antimicrobial activity of the poly herbal preparation was
quantified by determining the
MIC using the pour plate method Sanders (2012).
Mueller Hinton agar powder (26.6g) and bacteriological agar
powder (2.1g) were dissolved in 700ml of distilled water and the ingredients
were sterilized.
Two-fold serial dilutions were made by taking 6 ml from the
herbal preparation with an initial concentration of 250mg/ml and diluting with
6 ml of distilled water to obtain a dilution of 125mg/ml. The same procedure was followed to obtain the
dilution series of the poly herbal preparation. As a result, the poly herbal
extract with initial concentration of 250 mg/mL was
diluted to a final concentration
of 3. 906 mg/ml.
Molten MHA (20ml) was cooled to 500C and mixed
well with 3.0ml of each doubling dilutions (250 mg/ml, 125 mg/ml, 62.5 mg/ml.
31.25 mg/ml, 15.63 mg/ml,7.81 mg/ml, 3.91 mg/ml) and poured into sterile
culture plates and allowed
to dry. Inoculum was prepared as a suspension in a 0.9% normal
saline and the turbidity of the inoculum was adjusted to McFarland 0.5
standard. A grid containing 5 squares was drawn on the back of the glass
culture plates using marker pen. A volume of 5.0 µl of each suspension of organisms
(Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis,
Staphylococcus aureus) and the negative control (sterile
normal saline) were kept on the surface of the agar plate in each grid
using micropipette. Plates were then covered and left for overnight incubation
at 37◦C. The lowest concentration of the extract that inhibit the visible
growth of a microorganism after overnight incubation (when compared with the
control sterile normal saline) was determined as MIC and was carried out in triplicates
and average MIC was calculated Gunasekara et al. (2017).
2.9. STATISTICAL ANALYSIS
The results were
represented as the mean of three independent replicates ± standard deviation.
Statistical analysis of data was carried out by SPSS (Statistical
Package for Social Sciences) version 21.
3. RESULTS
3.1. ANTIMICROBIAL ACTIVITY OF THE POLY HERBAL
PREPARATION
The poly herbal
preparation has considerable antimicrobial activity against Staphylococcus
aureus (ATCC 25923) and Enterococcus faecalis (ATCC 29212) Table 1. It was unable to inhibit the growth of Pseudomonas
aeruginosa (ATCC 27853) and Escherichia
coli (ATCC25922). However, the preparation showed a lower zone of
inhibition for the tested bacteria in comparison to the standard antibiotic
(amoxicillin, penicillin, and gentamycin). Among the tested microorganism,
inhibitory activity of plant extract was found to be most active against Enterococcus
faecalis. Figure 2, Figure 3
Table 1
|
Table 1 The Zone of Inhibition of Poly Herbal Preparation (Mean ± SD) In Comparison with Standard Antibiotics |
||||
|
Antimicrobial agent |
Enterococcus
faecalis (ATCC
29212) |
Escherichia
coli (ATCC
25922) |
Pseudomonas
aeruginosa (ATCC
27853) |
Staphylococcus
aureus (ATCC
25923) |
|
Herbal preparation |
17.00 ± 1.00 |
0.33 ± 0.58 |
1.00 ± 0.00 |
16.00 ± 0.00 |
|
Amoxycillin |
39.33 ± 1.16 |
30.00 ± 0.00 |
15.00 ± 1.00 |
49.33 ± 1.16 |
|
Gentamycin |
25.00 ± 0.00 |
30.00 ± 0.00 |
38.33 ± 1.53 |
29.67 ± 0.58 |
|
Penicillin |
30.67 ± 1.16 |
23.33 ± 1.16 |
30.00 ± 0.00 |
50.00 ± 2.00 |
Figure 2
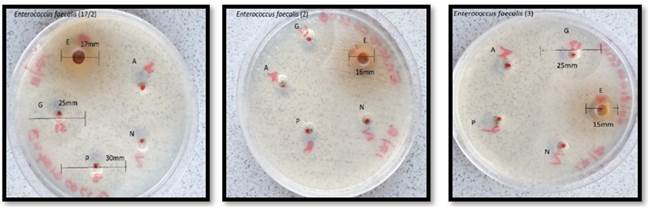
|
Figure 2 Triplicates of Enterococcus faecalis |
Figure 3
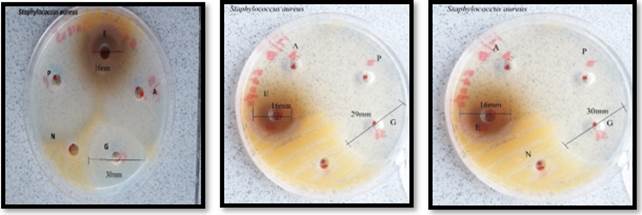
|
Figure 3 Triplicates of Staphylococcus
aureus P –
Penicillin N – Normal saline G –
Gentamicin A - Amoxicillin E -
Extract |
3.2. MIC OF THE POLY HERBAL PREPARATION
As depicted in the Table 2, the effective
concentration of herbal preparation for its maximum antimicrobial activity is
250mg/ml. In this concentration, the plant extract has inhibitory activity
against Pseudomonas aeruginosa, Enterococcus faecalis and Staphylococcus
aureus.
Table 2
|
Table 2 MIC of the Polyherbal Preparation |
|||||
|
Concentration
of herbal preparation (mg/ml) |
Escherichia
coli (ATCC
25922) |
(ATCC 27853) |
Enterococcus
faecalis (ATCC
29212) |
(ATCC
25923) |
Negative
control |
|
250 |
G |
NG |
NG |
NG |
G |
|
125 |
G |
G |
NG |
NG |
G |
|
62.5 |
G |
G |
G |
NG |
G |
|
31.25 |
G |
G |
G |
G |
G |
|
15.63 |
G |
G |
G |
G |
G |
|
7.81 |
G |
G |
G |
G |
G |
|
3.91 |
G |
G |
G |
G |
G |
|
G: Growth NG: No growth |
|||||
4. DISCUSSION
Although medicinal plants have been used from ancient times as natural remedies in Sri Lankan Ayurveda and are considered as alternatives to complement the synthetic medications, in-depth evidence based scientific investigations must be conducted to establish the scientific rationale for the efficacy. Thus, in this study, microbial susceptibility was evaluated for a reputed poly herbal preparation that is used as an effective treatment against periodontal disease and dental caries.
The study provides evidence to prove that the poly herbal formulation has moderate antibacterial action against the gram-positive bacteria Enterococcus faecalis, and Staphylococcus aureus that is responsible for oral mucosal diseases, periodontal disease, and dental caries.
The antimicrobial effect of individual ingredients of the
polyherbal formulation was confirmed by previous scientific research data. The indigenous medical literature has
stated that the extract of leaves from J.
officinale is used to treat mouth
ulcers due to its' vrana ropana (ulcer
healing), krimighna
(anti-microbial), sothaghna
(reduce swelling) and sophaghna (anti-inflammatory) actions Ayurveda Pharmacopoeia (2002). Another study has confirmed that the ethanolic
extracts of all part of the plant extract is effective against Staphylococcus
aureus, Enterococcus faecalis, Escherichia coli and Pseudomonas
aeruginosa Shahbaa and Al-Khazraji
(2015).
Raza
et al. (2010) have reported the antimicrobial activity of water
extract of T. chebula fruit
against Staphylococcus aureus Raza
et al. (2010). In another study it was revealed that
different solvent fractions of T. chebula fruit is effective against Staphylococcus aureus and Escherichia coli Bag et al. (2009). Further, the study has claimed
that Staphylococcus aureus strains
were found to be more susceptible to a hot aqueous extract and Escherichia coli strains to ethanol
extract Bag et al. (2009). It
has been reported that, T. chebula has the ability in decreasing
oral diseases, such as gingival inflammation, mouth ulcers and plaque formation
Gupta et al. (2016). The indigenous medical literature has also
made strong evidence reputing that it has krimighna, vranaropana, raktasthambhana
and sophagna
actions according to pharmacodynamics properties of T. chebula.
Crude methanol extract of T. cordifolia was reported to contained antibacterial activity against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans Singh et al. (2016). Phytochemical study of aqueous extract of T. cordifolia has confirmed that it contains alkaloids, flavonoids, glycosides and tannins Sharma et al. (2019). It further proved that the Ayurveda pharmacodynamics of sothahara, sophahara, raktasthambhana, vrana ropana and vrana shodana properties to be associated with T. cordifolia Ayurveda Pharmacopoeia (2002).
G. glabra was found to show potential antibacterial efficacy against primary plaque colonizers and periodontal pathogens Sharma et al. (2016). These results further confirmed its' sophahara, sothaghna, vrana sodhana, ropana and krimighna properties.
Several studies have reported that the crude extract of
the whole plant of D. triflorum to possess analgesic, anti-inflammatory, ulcer healing and anti-microbial
actions. Methanolic extract has shown considerable analgesic and
anti-inflammatory activities Liu
et al. (2013). Another study has
confirmed that methanolic extract has considerable anti-bacterial activity
against gram positive organisms such as Staphylococcus aureus and Pseudomonas aeruginosa Sharma
et al. (2013).
Therefore, the evidence based scientific data has
confirmed that the ingredients in the polyherbal preparation has an array of bioactivities
such as antimicrobial, anti-inflammatory, analgesic, and cleansing properties
thus serving as an effective remedy.
Further the existing literature significantly proved the pharmacological
actions mentioned in Ayurveda texts on each ingredient of poly herbal Gandusha
formulation to be effective against pathogens causing oral diseases. Moreover, this type of herbal preparation is much beneficial than antibiotics due
to the non -emergence of resistant by pathogens. They are cost effective and
therefore economically affordable. The plant materials are widely available in
abundance and easy preparation method has led to wide use among people who rely
on Ayurvedic treatments.
5. CONCLUSION
The poly herbal combination mentioned in Ayurveda consisting of J. officinale leaves, T. chebula fruits, T. cordifolia stem, D. triflorum whole plant and G. glabra roots has a potential for inhibiting oral pathogens. It therefore improve the oral hygiene and provide scientific rationale for its wide use among people who rely on Ayurvedic treatments. However, further studies especially in the clinical setting are required to confirm efficacy.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors are thankful to the Microbiology laboratory, Department of Medical Laboratory Sciences, Faculty of Allied Health Sciences, University of Sri Jayewardenepura, Sri Lanka, for providing their infrastructure and equipment for the study and all microbial cultures were supplied by the Quality Control Lab, Department of Microbiology, Medical Research Institute and the Department of Microbiology, National Hospital, Colombo 08, Sri Lanka.
REFERENCES
Agnivesha (2008). Charaka Samhita Revised by Charaka and Dhridabala
with the Ayurveda Dipika Commentary
of Chakrapanidatta V. Y. T. Acharya, C. S. Sansthan and Varanasi (Eds.) (5th
ed).
Ayurveda Pharmacopoeia (2002), Department of Ayurveda. Part Three, 1, 215-216, 305-306.
Bag, A., Bhattacharyya, S. K., Bharati, P., and Kumar, N. (2009). Evaluation of Antibacterial Properties of Chebulic Myrobalan (fruit of Terminalia chebula Retz.) Extracts Against Methicillin Resistant Staphylococcus Aureus and Trimethoprim-Sulphamethoxazole Resistant Uropathogenic Escherichia Coli. African Journal of Plant Science, 3(2), 25-29. https://doi.org/10.5897/AJPS.9000109.
Gunasekara, T., Radhika, N., Ragunathan, K. K., Gunathilaka, D., Weerasekera, M. M., Hewageegana, H., Arawwawala, L. A. D. M., and Fernando, S. (2017). Determination of Antimicrobial Potential of Five Herbs Used in Ayurveda Practices Against Candida Albicans, Candida Parapsilosis and Methicillin Resistant Staphylococcus Aureus. Ancient Science Of Life, 36(4), 187-190. https://doi.org/10.4103/asl.ASL_179_16.
Gupta, D., Gupta, R. K., Bhaskar, D. J., and Gupta, V. (2016). Comparative Evaluation of Terminalia Chebula Extract Mouthwash and Chlorhexidine Mouthwash on Plaque and Gingival Inflammation −4-week Randomised Control Trial. Oral Health and Preventive Dentistry, 14(3), 5-12. https://doi.org/10.3290/j.ohpd.a32994.
Kadam, A., Prasad, B. S., Bagadia, D., and Hiremath, V. R. (2011). Effect of Ayurvedic Herbs on Control Of Plaque And Gingivitis: A Randomized Controlled Trial. Ayu, 32(4), 532-535. https://doi.org/10.4103/0974-8520.96128.
Kanchan, N. M., Shubhangi, S. M., and Umesh, K. B. (2019). Determination of antibacterial activity of leaf extract of Jasminum officinale against oral pathogens in ulcer treatment. International Journal of Science and Research, 4(6), 235-247.
Khan, U. A., Rahman, H., Niaz, Z., Qasim, M., Khan, J., Tayyaba, and Rehman, B. (2013). Antibacterial Activity of Some Medicinal Plants Against Selected Human Pathogenic Bacteria. European Journal of Microbiology and Immunology, 3(4), 272-274. https://doi.org/10.1556/EuJMI.3.2013.4.6.
Liu, C., Wu, Y., Zhang, Q. J., Kang, W. Y., Zhang, L., and Zhou, Q. D. (2013). Advances in Studies on Chemical Constituents and Biological Activities of Desmodium Species. China Journal of Chinese Materia Medica, 38(23), 4006-4014.
Peiris, K. P. P., Wanigasekara, D. N., Sudesh, A. D. H., and Karunarathne, E. D. C. (2019). In-vitro Evaluation of Antimicrobial Efficacy of the Indigenous Formulation, Karna Bindu Against Common Pathogens Causing Otitis Infections. International Journal of Scientific Research and Innovative Technology, 7.
Raza, S. H., Mahayroookh, A. N., Rehman, A. B., and Ahmad, M. (2010). Evaluation of Antimicrobial Properties of Terminalia Chebula Retz. Pakistan Journal of Pharmacology, 27(1), 29-35. https://doi.org/10.4103/0974-8520.108859.
Sanders, E. R. J. (2012). Aseptic Laboratory Techniques : Plating methods. Visualized Experiments, 63, 3064. https://doi.org/10.3791/3064.
Shahbaa, M., and Al-Khazraji, A. (2015). Evaluation of Antibacterial Activity of Jasminum Officinale. International Journal of Pharmacy and Biological Sciences, 10(1), 121-124.
Sharangadhara. (2013). Sharangadhara Samhita of Pandita Sharangadhara with commentaries of Adhamalla Dipika and Kashirama Gudharth Dipika. Print P. P. Shastri and U. K. Vidyasagar (Eds.), 10/2-3. Chaukhamba Publication, 352.
Sharma, H., Yunus, G. Y., Mohapatra, A. K., Kulshrestha, R., Agrawal, R., and Kalra, M. (2016). Antimicrobial Efficacy of Three Medicinal Plants Glycyrrhiza Glabra, Ficus Religiosa, and Plantago Major on Inhibiting Primary Plaque Colonizers and Periodontal Pathogens: An in Vitro Study. Indian Journal of Dental Research, 27(2), 200-204. https://doi.org/10.4103/0970-9290.183135.
Sharma, P., Dwivedee, B. P., Bisht, D., Dash, A. K., and Kumar, D. (2019). The Chemical Constituents and Diverse Pharmacological Importance of Tinospora Cordifolia. Heliyon, 5(9), e02437. https://doi.org/10.1016/j.heliyon.2019.e02437.
Sharma, R., Parashar, B., and Kabra, A. (2013). Efficacy of Aqueous and Methanolic Extracts of Plant Desmodium for Potential Antibcterial Activity. International Journal of Pharmaceutical Sciences, 4(5), 1975-1981. http://dx.doi.org/10.13040/IJPSR.0975-8232.4(5).1975-81.
Singh, G., Saxena, R. K., and Singh, N. K. (2016). Screening of Potential Antimicrobial Activity of Indian Medicinal Plant of Different Solvent Extract : Tinospora Cordifolia and Hymenocallis Littoralis. International Research Journal of Engineering and Technology, 3, 928-932.
Singhal, G. D. (1972). Diagnostic Considerations in Ancient Indian Surgery Vol. III, Based on Nidana sthana of Susruta Samhita [16th Chapter]. Verse, 3. Chaukhamba Sanskrit Pratishthan, 231.
WHO Technical Report Series. (1995). Recent Advances in Oral Health. World Health Organization.
William, S., Maynard, K. H., and Barnet, M. L. (2001). A Text Book of Oral Pathology (4th ed). Harcourt Publishers India Private Limited.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.